Cellular prion protein transduces neuroprotective signals
- PMID: 12093733
- PMCID: PMC125390
- DOI: 10.1093/emboj/cdf324
Cellular prion protein transduces neuroprotective signals
Abstract
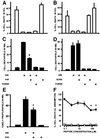
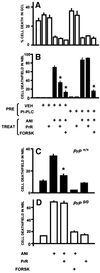
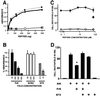
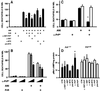
To test for a role for the cellular prion protein (PrP(c)) in cell death, we used a PrP(c)-binding peptide. Retinal explants from neonatal rats or mice were kept in vitro for 24 h, and anisomycin (ANI) was used to induce apoptosis. The peptide activated both cAMP/protein kinase A (PKA) and Erk pathways, and partially prevented cell death induced by ANI in explants from wild-type rodents, but not from PrP(c)-null mice. Neuroprotection was abolished by treatment with phosphatidylinositol-specific phospholipase C, with human peptide 106-126, with certain antibodies to PrP(c) or with a PKA inhibitor, but not with a MEK/Erk inhibitor. In contrast, antibodies to PrP(c) that increased cAMP also induced neuroprotection. Thus, engagement of PrP(c) transduces neuroprotective signals through a cAMP/PKA-dependent pathway. PrP(c) may function as a trophic receptor, the activation of which leads to a neuroprotective state.
Figures


Similar articles
-
Stress-inducible protein 1 is a cell surface ligand for cellular prion that triggers neuroprotection.EMBO J. 2002 Jul 1;21(13):3307-16. doi: 10.1093/emboj/cdf325. EMBO J. 2002. PMID: 12093732 Free PMC article.
-
Endocytosis of prion protein is required for ERK1/2 signaling induced by stress-inducible protein 1.J Neurosci. 2008 Jun 25;28(26):6691-702. doi: 10.1523/JNEUROSCI.1701-08.2008. J Neurosci. 2008. PMID: 18579743 Free PMC article.
-
Inhibition of T lymphocyte activation by cAMP is associated with down-regulation of two parallel mitogen-activated protein kinase pathways, the extracellular signal-related kinase and c-Jun N-terminal kinase.J Immunol. 1996 Aug 15;157(4):1514-22. J Immunol. 1996. PMID: 8759733
-
Role of cAMP-dependent protein kinase A activity in endothelial cell cytoskeleton rearrangement.Am J Physiol Lung Cell Mol Physiol. 2001 Jun;280(6):L1309-17. doi: 10.1152/ajplung.2001.280.6.L1309. Am J Physiol Lung Cell Mol Physiol. 2001. PMID: 11350812
-
C-terminal fragment of tetanus toxin heavy chain activates Akt and MEK/ERK signalling pathways in a Trk receptor-dependent manner in cultured cortical neurons.Biochem J. 2003 Jul 15;373(Pt 2):613-20. doi: 10.1042/BJ20030333. Biochem J. 2003. PMID: 12710887 Free PMC article.
Cited by
-
Truncated prion protein and Doppel are myelinotoxic in the absence of oligodendrocytic PrPC.J Neurosci. 2005 May 11;25(19):4879-88. doi: 10.1523/JNEUROSCI.0328-05.2005. J Neurosci. 2005. PMID: 15888663 Free PMC article.
-
Neurodegeneration induced by clustering of sialylated glycosylphosphatidylinositols of prion proteins.J Biol Chem. 2012 Mar 9;287(11):7935-44. doi: 10.1074/jbc.M111.275743. Epub 2012 Jan 19. J Biol Chem. 2012. PMID: 22262833 Free PMC article.
-
Proteolytic processing of the prion protein in health and disease.Am J Neurodegener Dis. 2012;1(1):15-31. Epub 2012 May 15. Am J Neurodegener Dis. 2012. PMID: 23383379 Free PMC article.
-
New molecular insights into cellular survival and stress responses: neuroprotective role of cellular prion protein (PrPC).Mol Neurobiol. 2007 Jun;35(3):236-44. doi: 10.1007/s12035-007-8003-y. Mol Neurobiol. 2007. PMID: 17917112 Review.
-
Cellular prion protein and caveolin-1 interaction in a neuronal cell line precedes Fyn/Erk 1/2 signal transduction.J Biomed Biotechnol. 2006;2006(5):69469. doi: 10.1155/JBB/2006/69469. J Biomed Biotechnol. 2006. PMID: 17489019 Free PMC article.
References
-
- Aguzzi A. and Weissmann,C. (1997) Prion research: the next frontiers. Nature, 389, 795–798. - PubMed
-
- Boquet D., Dery,O., Frobert,Y., Grassi,J. and Couraud,J.Y. (1995) Is hydropathic complementarity involved in antigen–antibody binding? Mol. Immunol., 32, 303–308. - PubMed
-
- Bounhar Y., Zhang,Y., Goodyear,C. and LeBlanc,A. (2001) Prion protein protects human neurons against Bax-mediated apoptosis. J. Biol. Chem., 276, 39145–39149. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

