Dystrophin Dp71 is critical for the clustered localization of potassium channels in retinal glial cells
- PMID: 12040037
- PMCID: PMC6758827
- DOI: 10.1523/JNEUROSCI.22-11-04321.2002
Dystrophin Dp71 is critical for the clustered localization of potassium channels in retinal glial cells
Abstract
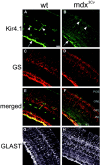
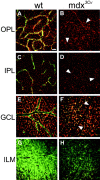
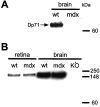
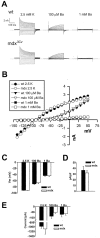
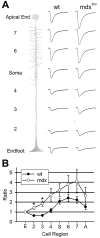
The Müller cell is the principal glial cell of the vertebrate retina. The primary conductance in Müller cells is the inwardly rectifying potassium channel Kir4.1 (BIR10 and KAB-2), which is highly concentrated at the endfeet at the vitreal border and to processes enveloping blood vessels. Such asymmetric and clustered distribution of Kir4.1 channels in Müller cells is thought to be critical for the buffering of extracellular potassium concentration in retina. Herein we investigated whether the distribution and functional properties of Kir4.1 channels are dependent on expression of the Dp71, a dystrophin isoform expressed in Müller cells. Kir4.1 distribution was determined in mouse retinal sections and whole mounts using anti-Kir4.1 antibodies and confocal microscopy. In Müller cells from wild-type mice, Kir4.1 is highly clustered in their endfeet and perivascular processes. In contrast, in Müller cells from the mdx(3Cv) mouse, which lacks the expression of Dp71, the Kir4.1 immunoreactivity is evenly distributed throughout the cell membrane. Surface expression of Kir4.1 is not affected in mdx(3Cv) Müller cells as current density of barium-sensitive inward currents in mdx(3Cv) Müller cells are not different from wild type. Focal extracellular potassium increases in isolated Müller cells shows that Kir channels in the mdx(3Cv) cells, as opposed to wild type, are less prominently concentrated in their endfeet. In summary, our data indicate that Dp71 is critical for the clustering but not membrane expression of Kir4.1 in mouse Müller cells. These results point to a new role for dystrophin in glial cells.
Figures
Similar articles
-
Kir4.1 and AQP4 associate with Dp71- and utrophin-DAPs complexes in specific and defined microdomains of Müller retinal glial cell membrane.Glia. 2008 Apr 15;56(6):597-610. doi: 10.1002/glia.20633. Glia. 2008. PMID: 18286645
-
Potassium channel Kir4.1 macromolecular complex in retinal glial cells.Glia. 2006 Jan 15;53(2):124-31. doi: 10.1002/glia.20271. Glia. 2006. PMID: 16206160
-
Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: phenotypic impact in retina.J Neurosci. 2000 Aug 1;20(15):5733-40. doi: 10.1523/JNEUROSCI.20-15-05733.2000. J Neurosci. 2000. PMID: 10908613 Free PMC article.
-
Molecular substrates of potassium spatial buffering in glial cells.Mol Neurobiol. 2003 Oct;28(2):195-208. doi: 10.1385/MN:28:2:195. Mol Neurobiol. 2003. PMID: 14576456 Review.
-
Inwardly rectifying potassium channels (Kir) in central nervous system glia: a special role for Kir4.1 in glial functions.J Cell Mol Med. 2006 Jan-Mar;10(1):33-44. doi: 10.1111/j.1582-4934.2006.tb00289.x. J Cell Mol Med. 2006. PMID: 16563220 Free PMC article. Review.
Cited by
-
Biochemical and Functional Interplay Between Ion Channels and the Components of the Dystrophin-Associated Glycoprotein Complex.J Membr Biol. 2018 Aug;251(4):535-550. doi: 10.1007/s00232-018-0036-9. Epub 2018 May 19. J Membr Biol. 2018. PMID: 29779049 Review.
-
Visual impairment in the absence of dystroglycan.J Neurosci. 2009 Oct 21;29(42):13136-46. doi: 10.1523/JNEUROSCI.0474-09.2009. J Neurosci. 2009. PMID: 19846701 Free PMC article.
-
The Membrane Properties of Cochlear Root Cells are Consistent with Roles in Potassium Recirculation and Spatial Buffering.J Assoc Res Otolaryngol. 2010 Sep;11(3):435-48. doi: 10.1007/s10162-010-0218-3. Epub 2010 Apr 15. J Assoc Res Otolaryngol. 2010. PMID: 20393778 Free PMC article.
-
Dystrophin Short Product, Dp71, Interacts with AQP4 and Kir4.1 Channels in the Mouse Cerebellar Glial Cells in Contrast to Dp427 at Inhibitory Postsynapses in the Purkinje Neurons.Mol Neurobiol. 2023 Jul;60(7):3664-3677. doi: 10.1007/s12035-023-03296-w. Epub 2023 Mar 15. Mol Neurobiol. 2023. PMID: 36918517
-
Evidence of the involvement of dystrophin Dp71 in corneal angiogenesis.Mol Vis. 2019 Nov 14;25:714-721. eCollection 2019. Mol Vis. 2019. PMID: 31814696 Free PMC article.
References
-
- Aleman V, Osorio B, Chavez O, Rendon A, Mornet D, Martinez D. Subcellular localization of Dp71 dystrophin isoforms in cultured hippocampal neurons and forebrain astrocytes. Histochem Cell Biol. 2001;115:243–254. - PubMed
-
- Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, WuZ, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. - PubMed
-
- Bringmann A, Francke M, Pannicke T, Biedermann B, Faude F, Enzmann V, Wiedemann P, Reichelt W, Reichenbach A. Human Müller glial cells: altered potassium channel activity in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1999;40:3316–3323. - PubMed
-
- Cibis GW, Fitzgerald KM, Harris DJ, Rothberg PG, Rupani M. The effects of dystrophin gene mutations on the ERG in mice and humans. Invest Ophthalmol Vis Sci. 1993;34:3646–3652. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

