Synergistic activation of the rat laminin gamma1 chain promoter by the gut-enriched Kruppel-like factor (GKLF/KLF4) and Sp1
- PMID: 12034813
- PMCID: PMC117209
- DOI: 10.1093/nar/30.11.2270
Synergistic activation of the rat laminin gamma1 chain promoter by the gut-enriched Kruppel-like factor (GKLF/KLF4) and Sp1
Abstract
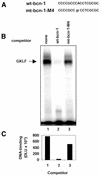
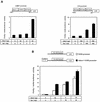
Laminin is a multifunctional heterotrimeric protein present in extracellular matrix where it regulates processes that compose tissue architecture including cell differentiation. Laminin gamma1 is the most widely expressed laminin chain and its absence causes early lethality in mouse embryos. Laminin gamma1 chain gene (LAMC1) promoter contains several GC/GT-rich motifs including the bcn-1 element. Using the bcn-1 element as a bait in the yeast one-hybrid screen, we cloned the gut-enriched Kruppel-like factor (GKLF or KLF4) from a rat mesangial cell library. We show that GKLF binds bcn-1, but this binding is not required for the GKLF-mediated activation of the LAMC1 promoter. The activity of GKLF is dependent on a synergism with another Kruppel-like factor, Sp1. The LAMC1 promoter appears to have multiple GKLF- and Sp1-responsive elements which may account for the synergistic activation. We provide evidence that the synergistic action of GKLF and Sp1 is dependent on the promoter context and the integrity of GKLF activation and DNA-binding domain. GKLF is thought to participate in the switch from cell proliferation to differentiation. Thus, the Sp1-GKLF synergistic activation of the LAMC1 promoter may be one of the avenues for expression of laminin gamma1 chain when laminin is needed to regulate cell differentiation.
Figures


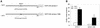



Similar articles
-
Gut-enriched Krüppel-like factor represses cyclin D1 promoter activity through Sp1 motif.Nucleic Acids Res. 2000 Aug 1;28(15):2969-76. doi: 10.1093/nar/28.15.2969. Nucleic Acids Res. 2000. PMID: 10908361 Free PMC article.
-
The gut-enriched Krüppel-like factor suppresses the activity of the CYP1A1 promoter in an Sp1-dependent fashion.J Biol Chem. 1998 Jul 10;273(28):17917-25. doi: 10.1074/jbc.273.28.17917. J Biol Chem. 1998. PMID: 9651398 Free PMC article.
-
Sp1-mediated transactivation of LamC1 promoter and coordinated expression of laminin-gamma1 and Sp1 in human hepatocellular carcinomas.Am J Pathol. 1997 Dec;151(6):1663-72. Am J Pathol. 1997. PMID: 9403717 Free PMC article.
-
Sp1- and Krüppel-like transcription factors.Genome Biol. 2003;4(2):206. doi: 10.1186/gb-2003-4-2-206. Epub 2003 Feb 3. Genome Biol. 2003. PMID: 12620113 Free PMC article. Review.
-
A tale of three fingers: the family of mammalian Sp/XKLF transcription factors.Nucleic Acids Res. 1999 Aug 1;27(15):2991-3000. doi: 10.1093/nar/27.15.2991. Nucleic Acids Res. 1999. PMID: 10454592 Free PMC article. Review.
Cited by
-
Regulation of mouse lens maturation and gene expression by Krüppel-like factor 4.Exp Eye Res. 2013 Nov;116:205-18. doi: 10.1016/j.exer.2013.09.010. Epub 2013 Sep 25. Exp Eye Res. 2013. PMID: 24076321 Free PMC article.
-
Down-regulation of gut-enriched Kruppel-like factor expression in esophageal cancer.World J Gastroenterol. 2002 Dec;8(6):966-70. doi: 10.3748/wjg.v8.i6.966. World J Gastroenterol. 2002. PMID: 12439907 Free PMC article.
-
Convergence of the thyroid hormone and gut-enriched Krüppel-like factor pathways in the context of enterocyte differentiation.J Gastrointest Surg. 2003 Dec;7(8):1053-61; discussion 1061. doi: 10.1016/j.gassur.2003.09.006. J Gastrointest Surg. 2003. PMID: 14675715
-
LAMC1 upregulation via TGFβ induces inflammatory cancer-associated fibroblasts in esophageal squamous cell carcinoma via NF-κB-CXCL1-STAT3.Mol Oncol. 2021 Nov;15(11):3125-3146. doi: 10.1002/1878-0261.13053. Epub 2021 Jul 22. Mol Oncol. 2021. PMID: 34218518 Free PMC article.
-
Development potential of extracellular matrix hydrogels as hemostatic materials.Front Bioeng Biotechnol. 2023 Jun 13;11:1187474. doi: 10.3389/fbioe.2023.1187474. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37383519 Free PMC article. Review.
References
-
- Tunggal P., Smyth,N., Paulsson,M. and Ott,M.C. (2000) Laminins: structure and genetic regulation. Microsc. Res. Tech., 51, 214–227. - PubMed
-
- Hansen K. and Abrass,C.K. (1999) Role of laminin isoforms in glomerular structure. Pathobiology, 67, 84–91. - PubMed
-
- Deutzmann R. (1995) Laminin. In Schlondorff,D. and Bonventre,J.V. (eds), Molecular Nephrology. Kidney Function in Health and Disease. Dekker, New York, pp. 41–59.
-
- Simon-Assmann P., Lefebvre,O., Bellissent-Waydelich,A., Olsen,J., Orian-Rousseau,V. and De Arcangelis,A. (1998) The laminins: role in intestinal morphogenesis and differentiation. Ann. N. Y. Acad. Sci., 859, 46–64. - PubMed
-
- Gudas L.J., Grippo,J.F., Kim,K.W., Larosa,G.J. and Stoner,C.M. (1990) The regulation of the expression of genes encoding basement membrane proteins during the retinoic-acid associated differentiation of murine teratocarcinoma cells. Ann. N. Y. Acad. Sci., 580, 245–254. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

