LIM kinase and Diaphanous cooperate to regulate serum response factor and actin dynamics
- PMID: 12034774
- PMCID: PMC2173419
- DOI: 10.1083/jcb.200203126
LIM kinase and Diaphanous cooperate to regulate serum response factor and actin dynamics
Abstract
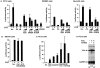
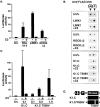
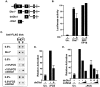
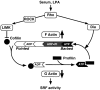
The small GTPase RhoA controls activity of serum response factor (SRF) by inducing changes in actin dynamics. We show that in PC12 cells, activation of SRF after serum stimulation is RhoA dependent, requiring both actin polymerization and the Rho kinase (ROCK)-LIM kinase (LIMK)-cofilin signaling pathway, previously shown to control F-actin turnover. Activation of SRF by overexpression of wild-type LIMK or ROCK-insensitive LIMK mutants also requires functional RhoA, indicating that a second RhoA-dependent signal is involved. This is provided by the RhoA effector mDia: dominant interfering mDia1 derivatives inhibit both serum- and LIMK-induced SRF activation and reduce the ability of LIMK to induce F-actin accumulation. These results demonstrate a role for LIMK in SRF activation, and functional cooperation between RhoA-controlled LIMK and mDia effector pathways.
Figures


Similar articles
-
The diaphanous-related formin mDia1 controls serum response factor activity through its effects on actin polymerization.Mol Biol Cell. 2002 Nov;13(11):4088-99. doi: 10.1091/mbc.02-06-0092. Mol Biol Cell. 2002. PMID: 12429848 Free PMC article.
-
A role for VASP in RhoA-Diaphanous signalling to actin dynamics and SRF activity.EMBO J. 2003 Jun 16;22(12):3050-61. doi: 10.1093/emboj/cdg287. EMBO J. 2003. PMID: 12805219 Free PMC article.
-
Rho-ROCK-LIMK-cofilin pathway regulates shear stress activation of sterol regulatory element binding proteins.Circ Res. 2003 Jun 27;92(12):1296-304. doi: 10.1161/01.RES.0000078780.65824.8B. Epub 2003 May 29. Circ Res. 2003. PMID: 12775580
-
Lim kinases, regulators of actin dynamics.Int J Biochem Cell Biol. 2007;39(6):1071-6. doi: 10.1016/j.biocel.2006.11.011. Epub 2006 Nov 28. Int J Biochem Cell Biol. 2007. PMID: 17188549 Review.
-
LIM-kinase as a regulator of actin dynamics in spermatogenesis.Cytogenet Genome Res. 2003;103(3-4):290-8. doi: 10.1159/000076815. Cytogenet Genome Res. 2003. PMID: 15051950 Review.
Cited by
-
Drosophila homologue of Diaphanous 1 (DIAPH1) controls the metastatic potential of colon cancer cells by regulating microtubule-dependent adhesion.Oncotarget. 2015 Jul 30;6(21):18577-89. doi: 10.18632/oncotarget.4094. Oncotarget. 2015. PMID: 26124177 Free PMC article.
-
Megakaryoblastic leukemia 1, a potent transcriptional coactivator for serum response factor (SRF), is required for serum induction of SRF target genes.Mol Cell Biol. 2003 Sep;23(18):6597-608. doi: 10.1128/MCB.23.18.6597-6608.2003. Mol Cell Biol. 2003. PMID: 12944485 Free PMC article.
-
Control of vascular smooth muscle function by Src-family kinases and reactive oxygen species in health and disease.J Physiol. 2015 Sep 1;593(17):3815-28. doi: 10.1113/jphysiol.2014.285304. Epub 2014 Dec 1. J Physiol. 2015. PMID: 25384773 Free PMC article. Review.
-
Actin-dependent activation of serum response factor in T cells by the viral oncoprotein tip.Cell Commun Signal. 2012 Mar 3;10(1):5. doi: 10.1186/1478-811X-10-5. Cell Commun Signal. 2012. PMID: 22385615 Free PMC article.
-
Human CNK1 acts as a scaffold protein, linking Rho and Ras signal transduction pathways.Mol Cell Biol. 2004 Feb;24(4):1736-46. doi: 10.1128/MCB.24.4.1736-1746.2004. Mol Cell Biol. 2004. PMID: 14749388 Free PMC article.
References
-
- Alberts, A.S. 2001. Identification of a carboxy-terminal diaphanous-related formin homology protein autoregulatory domain. J. Biol. Chem. 276:2824–2830. - PubMed
-
- Amano, M., M. Ito, K. Kimura, Y. Fukata, K. Chihara, T. Nakano, Y. Matsuura, and K. Kaibuchi. 1996. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 271:20246–20249. - PubMed
-
- Amano, M., Y. Fukata, and K. Kaibuchi. 2000. Regulation and functions of Rho-associated kinase. Exp. Cell Res. 261:44–51. - PubMed
-
- Arber, S., F.A. Barbayannis, H. Hanser, C. Schneider, C.A. Stanyon, O. Bernard, and P. Caroni. 1998. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 393:805–809. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

