Role of SHP-2 in fibroblast growth factor receptor-mediated suppression of myogenesis in C2C12 myoblasts
- PMID: 11997521
- PMCID: PMC133814
- DOI: 10.1128/MCB.22.11.3875-3891.2002
Role of SHP-2 in fibroblast growth factor receptor-mediated suppression of myogenesis in C2C12 myoblasts
Abstract
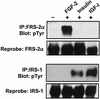
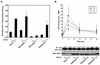
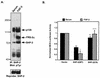
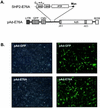
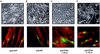
Ligand activation of the fibroblast growth factor receptor (FGFR) represses myogenesis and promotes activation of extracellular signal-regulated kinases 1 and 2 (Erks). The precise mechanism through which the FGFR transmits both of these signals in myoblasts remains unclear. The SH2 domain-containing protein tyrosine phosphatase, SHP-2, has been shown to participate in the regulation of FGFR signaling. However, no role for SHP-2 in FGFR myogenic signaling is known. In this study, we show that stimulation of C2C12 myoblasts with FGF-2 induces SHP-2 complex formation with tyrosyl-phosphorylated FGFR substrate 2 alpha (FRS-2 alpha). Both the catalytic activity and, to a much lesser extent, the Grb2 binding-tyrosyl phosphorylation sites of SHP-2 are required for maximal FGF-2-induced Erk activity and Elk-1 transactivation. When overexpressed in C2C12 myoblasts, wild-type SHP-2, but not a catalytically inactive SHP-2 mutant, potentiates the suppressive effects of FGF-2 on muscle-specific gene expression. In addition, expression of a constitutively active mutant of SHP-2 is sufficient to prevent myogenesis. The constitutively active mutant of SHP-2 induces hyper-tyrosyl phosphorylation of FRS-2 alpha but fails to stimulate or potentiate either FGF-2-induced Erk activation or Elk-1 transactivation. These data suggest that in myoblasts, SHP-2 represses myogenesis via a pathway that is independent of the Erks. We propose that SHP-2 plays a pivotal role in FGFR signaling in myoblasts via both Erk-dependent and Erk-independent pathways.
Figures





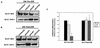

Similar articles
-
SHP-2 complex formation with the SHP-2 substrate-1 during C2C12 myogenesis.J Cell Sci. 2001 Jun;114(Pt 11):2187-98. doi: 10.1242/jcs.114.11.2187. J Cell Sci. 2001. PMID: 11493654
-
The major vault protein is a novel substrate for the tyrosine phosphatase SHP-2 and scaffold protein in epidermal growth factor signaling.J Biol Chem. 2004 Jul 9;279(28):29374-85. doi: 10.1074/jbc.M313955200. Epub 2004 May 7. J Biol Chem. 2004. PMID: 15133037
-
The Shp-2 tyrosine phosphatase has opposite effects in mediating the activation of extracellular signal-regulated and c-Jun NH2-terminal mitogen-activated protein kinases.J Biol Chem. 1998 Feb 27;273(9):4904-8. doi: 10.1074/jbc.273.9.4904. J Biol Chem. 1998. PMID: 9478933
-
Negative Regulation of FGFR (Fibroblast Growth Factor Receptor) Signaling.Cells. 2021 May 28;10(6):1342. doi: 10.3390/cells10061342. Cells. 2021. PMID: 34071546 Free PMC article. Review.
-
Multiple roles of RBM4 in muscle cell differentiation.Front Biosci (Schol Ed). 2012 Jan 1;4(1):181-9. doi: 10.2741/260. Front Biosci (Schol Ed). 2012. PMID: 22202052 Review.
Cited by
-
Noonan syndrome-associated SHP-2/Ptpn11 mutants enhance SIRPalpha and PZR tyrosyl phosphorylation and promote adhesion-mediated ERK activation.J Biol Chem. 2008 May 30;283(22):15328-38. doi: 10.1074/jbc.M801382200. Epub 2008 Mar 31. J Biol Chem. 2008. PMID: 18378677 Free PMC article.
-
SH2 Domain-Containing Phosphatase-2 Is a Novel Antifibrotic Regulator in Pulmonary Fibrosis.Am J Respir Crit Care Med. 2017 Feb 15;195(4):500-514. doi: 10.1164/rccm.201602-0329OC. Am J Respir Crit Care Med. 2017. PMID: 27736153 Free PMC article.
-
HIF-1α Dependent Wound Healing Angiogenesis In Vivo Can Be Controlled by Site-Specific Lentiviral Magnetic Targeting of SHP-2.Mol Ther. 2017 Jul 5;25(7):1616-1627. doi: 10.1016/j.ymthe.2017.04.007. Epub 2017 Apr 20. Mol Ther. 2017. PMID: 28434868 Free PMC article.
-
Novel role for SHP-2 in nutrient-responsive control of S6 kinase 1 signaling.Mol Cell Biol. 2013 Jan;33(2):293-306. doi: 10.1128/MCB.01285-12. Epub 2012 Nov 5. Mol Cell Biol. 2013. PMID: 23129808 Free PMC article.
-
Transcriptional profiling of mesenchymal stromal cells from young and old rats in response to Dexamethasone.BMC Genomics. 2006 Apr 27;7:95. doi: 10.1186/1471-2164-7-95. BMC Genomics. 2006. PMID: 16643645 Free PMC article.
References
-
- Adachi, M., M. Sekiya, T. Miyachi, K. Matsuno, Y. Hinoda, K. Imai, and A. Yachi. 1992. Molecular cloning of a novel protein-tyrosine phosphatase, SH-PTP3, with sequence similarity to the src-homology region 2. FEBS Lett. 314:335-339. - PubMed
-
- Bennett, A. M., and N. K. Tonks. 1997. Regulation of distinct stages of skeletal muscle differentiation by mitogen-activated protein kinases. Science 278:1288-1291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
