Identification and characterization of L-selectin ligands in porcine lymphoid tissues
- PMID: 11985664
- PMCID: PMC1782687
- DOI: 10.1046/j.1365-2567.2002.01393.x
Identification and characterization of L-selectin ligands in porcine lymphoid tissues
Abstract
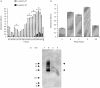
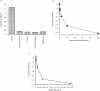
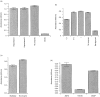
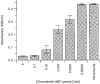
A human L-selectin-ZZ fusion protein was used to screen porcine inguinal lymph nodes for the presence of monoclonal antibody (mAb) MECA 79-negative ligands. Fractionation of lymph node-conditioned medium by anion-exchange chromatography revealed two distinct L-selectin-binding fractions, one containing a MECA 79 non-reactive species and the second containing two MECA 79 reactive species of approximately 84 000 and 210 000 molecular weight. The MECA 79 non-reactive species exhibited Ca2+- and lectin-dependent binding to L-selectin-ZZ in a solid-phase capture enzyme-linked immunosorbent assay (ELISA), and this was specifically disrupted by the addition of EDTA, mannose-6-phosphate and the blocking anti-L-selectin mAb, DREG-56. Enzymatic characterization of the ligand by trypsin, O-sialoglycoprotease endopeptidase, heparinases I and III, or chondroitinase ABC lyase digestion indicated that L-selectin binding was predominantly dependent upon a chondroitin sulphate-modified glycoprotein determinant. Although Coomassie Blue staining of sodium dodecyl sulphate (SDS) polyacrylamide gels did not reveal a detectable protein species, carbohydrate-specific staining using GlycoTrack revealed a single, heavily glycosylated protein of high molecular weight (> 220 000). These studies have revealed the existence of a MECA 79 non-reactive, chondroitin sulphate glycosaminoglycan-modified ligand, termed csgp>220, which is secreted by peripheral lymph nodes and may play a role in leucocyte trafficking to the lymph node.
Figures

Similar articles
-
Sulfation and sialylation requirements for a glycoform of CD34, a major endothelial ligand for L-selectin in porcine peripheral lymph nodes.Glycobiology. 1997 Mar;7(2):305-14. doi: 10.1093/glycob/7.2.305. Glycobiology. 1997. PMID: 9134437
-
Sulfation-dependent recognition of high endothelial venules (HEV)-ligands by L-selectin and MECA 79, and adhesion-blocking monoclonal antibody.J Exp Med. 1994 Dec 1;180(6):2219-26. doi: 10.1084/jem.180.6.2219. J Exp Med. 1994. PMID: 7525849 Free PMC article.
-
Complexity and differential expression of carbohydrate epitopes associated with L-selectin recognition of high endothelial venules.Am J Pathol. 1998 Feb;152(2):469-77. Am J Pathol. 1998. PMID: 9466573 Free PMC article.
-
A novel endothelial L-selectin ligand activity in lymph node medulla that is regulated by alpha(1,3)-fucosyltransferase-IV.J Exp Med. 2003 Nov 3;198(9):1301-12. doi: 10.1084/jem.20030182. J Exp Med. 2003. PMID: 14597733 Free PMC article.
-
Sulfated L-selectin ligands as a therapeutic target in chronic inflammation.Trends Immunol. 2006 Dec;27(12):559-65. doi: 10.1016/j.it.2006.10.007. Epub 2006 Oct 17. Trends Immunol. 2006. PMID: 17049924 Review.
Cited by
-
Human L-selectin preferentially binds synthetic glycosulfopeptides modeled after endoglycan and containing tyrosine sulfate residues and sialyl Lewis x in core 2 O-glycans.Glycobiology. 2010 Sep;20(9):1170-85. doi: 10.1093/glycob/cwq083. Epub 2010 May 27. Glycobiology. 2010. PMID: 20507883 Free PMC article.
-
L-Selectin ligands in lymphoid tissues and models of inflammation.Inflammation. 2003 Oct;27(5):265-80. doi: 10.1023/a:1026056525755. Inflammation. 2003. PMID: 14635784 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

