Epidermolysis bullosa and embryonic lethality in mice lacking the multi-PDZ domain protein GRIP1
- PMID: 11983858
- PMCID: PMC124486
- DOI: 10.1073/pnas.092130099
Epidermolysis bullosa and embryonic lethality in mice lacking the multi-PDZ domain protein GRIP1
Abstract
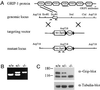
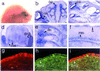
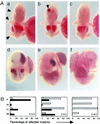
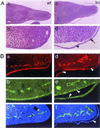
Glutamate receptor-interacting protein 1 (GRIP1) is an adaptor protein composed of seven PDZ (postsynaptic density-95/Discs large/zona occludens-1) domains, capable of mediating diverse protein-protein interactions. GRIP1 has been implicated in the regulation of neuronal synaptic function, but its physiologic roles have not been defined in vivo. We find that elimination of murine GRIP1 results in embryonic lethality. GRIP1(-/-) embryos develop abnormalities of the dermo-epidermal junction, resulting in extensive skin blistering around day 12 of embryonic life. Ultra-structural characterization of the blisters (or bullae) revealed cleavage of the dermo-epidermal junction below the lamina densa, an alteration reminiscent of the dystrophic form of human epidermolysis bullosa. Blisters were also observed in the lateral ventricle of the brain and in the meninges covering the cerebral cortex. These genetic data suggest that the GRIP1 scaffolding protein is required for the formation and integrity of the dermo-epidermal junction and reveal the importance of PDZ domains in the organization of supramolecular structures essential for mammalian embryonic development.
Figures


Similar articles
-
A direct functional link between the multi-PDZ domain protein GRIP1 and the Fraser syndrome protein Fras1.Nat Genet. 2004 Feb;36(2):172-7. doi: 10.1038/ng1292. Epub 2004 Jan 18. Nat Genet. 2004. PMID: 14730302
-
Review of collagen VII sequence variants found in Australasian patients with dystrophic epidermolysis bullosa reveals nine novel COL7A1 variants.J Dermatol Sci. 2007 Jun;46(3):169-78. doi: 10.1016/j.jdermsci.2007.02.006. Epub 2007 Apr 10. J Dermatol Sci. 2007. PMID: 17425959 Review.
-
Dystrophic epidermolysis bullosa phenotypes in a large consanguineous Tunisian family.J Dermatol Sci. 2009 May;54(2):114-20. doi: 10.1016/j.jdermsci.2009.01.006. Epub 2009 Mar 3. J Dermatol Sci. 2009. PMID: 19261445
-
Restoration of type VII collagen expression and function in dystrophic epidermolysis bullosa.Nat Genet. 2002 Dec;32(4):670-5. doi: 10.1038/ng1041. Epub 2002 Nov 11. Nat Genet. 2002. PMID: 12426566
-
Mutation analysis and characterization of COL7A1 mutations in dystrophic epidermolysis bullosa.Exp Dermatol. 2008 Jul;17(7):553-68. doi: 10.1111/j.1600-0625.2008.00723.x. Exp Dermatol. 2008. PMID: 18558993 Review.
Cited by
-
Osteopoikilosis, short stature and mental retardation as key features of a new microdeletion syndrome on 12q14.J Med Genet. 2007 Apr;44(4):264-8. doi: 10.1136/jmg.2006.047860. Epub 2007 Jan 12. J Med Genet. 2007. PMID: 17220210 Free PMC article.
-
The glutamate receptor-interacting protein family of GluR2-binding proteins is required for long-term synaptic depression expression in cerebellar Purkinje cells.J Neurosci. 2008 May 28;28(22):5752-5. doi: 10.1523/JNEUROSCI.0654-08.2008. J Neurosci. 2008. PMID: 18509036 Free PMC article.
-
NMDA receptors in GABAergic synapses during postnatal development.PLoS One. 2012;7(5):e37753. doi: 10.1371/journal.pone.0037753. Epub 2012 May 25. PLoS One. 2012. PMID: 22662211 Free PMC article.
-
Breakdown of the reciprocal stabilization of QBRICK/Frem1, Fras1, and Frem2 at the basement membrane provokes Fraser syndrome-like defects.Proc Natl Acad Sci U S A. 2006 Aug 8;103(32):11981-6. doi: 10.1073/pnas.0601011103. Epub 2006 Jul 31. Proc Natl Acad Sci U S A. 2006. PMID: 16880404 Free PMC article.
-
Glutamate Receptor Interacting Protein 1 Regulates CD4(+) CTLA-4 Expression and Transplant Rejection.Am J Transplant. 2016 May;16(5):1383-93. doi: 10.1111/ajt.13623. Epub 2016 Feb 25. Am J Transplant. 2016. PMID: 26601915 Free PMC article.
References
-
- Pawson T, Scott J D. Science. 1997;278:2075–2080. - PubMed
-
- Pawson T, Nash P. Genes Dev. 2000;14:1027–1047. - PubMed
-
- Sheng M, Sala C. Annu Rev Neurosci. 2001;24:1–29. - PubMed
-
- Kim E, Niethammer M, Rothschild A, Jan Y N, Sheng M. Nature (London) 1995;378:85–88. - PubMed
-
- Kornau H C, Schenker L T, Kennedy M B, Seeburg P H. Science. 1995;269:1737–1740. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

