AMP-activated kinase regulates cytoplasmic HuR
- PMID: 11971974
- PMCID: PMC133799
- DOI: 10.1128/MCB.22.10.3425-3436.2002
AMP-activated kinase regulates cytoplasmic HuR
Abstract
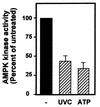
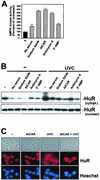
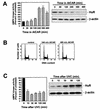
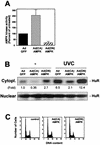
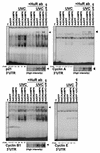
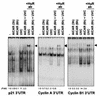
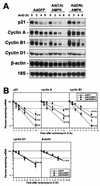
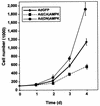
While transport of RNA-binding protein HuR from nucleus to cytoplasm is emerging as a key regulatory step for HuR function, the mechanisms underlying this process remain poorly understood. Here, we report that the AMP-activated kinase (AMPK), an enzyme involved in responding to metabolic stresses, potently regulates the levels of cytoplasmic HuR. Inhibition of AMPK, accomplished either through cell treatment or by adenovirus infection to express dominant-negative AMPK, was found to increase the level of HuR in the cytoplasm and to enhance the binding of HuR to p21, cyclin B1, and cyclin A mRNA transcripts and elevate their expression and half-lives. Conversely, AMPK activation, achieved by means including infection to express constitutively active AMPK, resulted in reduced cytoplasmic HuR; decreased levels and half-lives of mRNAs encoding p21, cyclin A, and cyclin B1; and diminished HuR association with the corresponding transcripts. We therefore propose a novel function for AMPK as a regulator of cytoplasmic HuR levels, which in turn influences the mRNA-stabilizing function of HuR and the expression of HuR target transcripts.
Figures




Similar articles
-
Increased AMP:ATP ratio and AMP-activated protein kinase activity during cellular senescence linked to reduced HuR function.J Biol Chem. 2003 Jul 18;278(29):27016-23. doi: 10.1074/jbc.M300318200. Epub 2003 May 1. J Biol Chem. 2003. PMID: 12730239
-
Polyamines modulate the subcellular localization of RNA-binding protein HuR through AMP-activated protein kinase-regulated phosphorylation and acetylation of importin alpha1.Biochem J. 2008 Jan 15;409(2):389-98. doi: 10.1042/BJ20070860. Biochem J. 2008. PMID: 17919121 Free PMC article.
-
AMP-activated protein kinase-regulated phosphorylation and acetylation of importin alpha1: involvement in the nuclear import of RNA-binding protein HuR.J Biol Chem. 2004 Nov 12;279(46):48376-88. doi: 10.1074/jbc.M409014200. Epub 2004 Sep 1. J Biol Chem. 2004. PMID: 15342649
-
Signalling pathways regulating nucleo-cytoplasmic shuttling of the mRNA-binding protein HuR.Cell Signal. 2008 Dec;20(12):2165-73. doi: 10.1016/j.cellsig.2008.05.007. Epub 2008 May 23. Cell Signal. 2008. PMID: 18585896 Review.
-
HuR, a key post-transcriptional regulator, and its implication in progression of breast cancer.Histol Histopathol. 2010 Oct;25(10):1331-40. doi: 10.14670/HH-25.1331. Histol Histopathol. 2010. PMID: 20712017 Review.
Cited by
-
Activation of AMP-activated protein kinase inhibits the proliferation of human endothelial cells.J Pharmacol Exp Ther. 2012 Sep;342(3):827-34. doi: 10.1124/jpet.112.194712. Epub 2012 Jun 13. J Pharmacol Exp Ther. 2012. PMID: 22700432 Free PMC article.
-
Inhibition of translocator protein 18 kDa suppressed the progression of glioma via the ELAV-like RNA-binding protein 1/MAPK-activated protein kinase 3 axis.Bioengineered. 2022 Mar;13(3):7457-7470. doi: 10.1080/21655979.2022.2048992. Bioengineered. 2022. PMID: 35285415 Free PMC article.
-
Hypoxia reduces HNF4α/MODY1 protein expression in pancreatic β-cells by activating AMP-activated protein kinase.J Biol Chem. 2017 May 26;292(21):8716-8728. doi: 10.1074/jbc.M116.767574. Epub 2017 Mar 31. J Biol Chem. 2017. PMID: 28364040 Free PMC article.
-
Loss of CARM1 is linked to reduced HuR function in replicative senescence.BMC Mol Biol. 2013 Jul 9;14:15. doi: 10.1186/1471-2199-14-15. BMC Mol Biol. 2013. PMID: 23837869 Free PMC article.
-
Protein kinase C alpha-dependent phosphorylation of the mRNA-stabilizing factor HuR: implications for posttranscriptional regulation of cyclooxygenase-2.Mol Biol Cell. 2007 Jun;18(6):2137-48. doi: 10.1091/mbc.e06-09-0850. Epub 2007 Mar 28. Mol Biol Cell. 2007. PMID: 17392515 Free PMC article.
References
-
- Atasoy, U., J. Watson, D. Patel, and J. D. Keene. 1998. ELAV protein HuA (HuR) can redistribute between nucleus and cytoplasm and is upregulated during serum stimulation and T cell activation. J. Cell Sci. 111:3145-3156. - PubMed
-
- Bohjanen, P. R., B. Petryniak, C. H. June, C. B. Thompson, and T. Lindsten. 1992. AU RNA-binding factors differ in their binding specificities and affinities. J. Biol. Chem. 267:6302-6309. - PubMed
-
- Bost, F., N. Dean, R. McKay, and D. Mercola. 1997. The JUN kinase/stress-activated protein kinase pathway is required for epidermal growth factor stimulation of growth of human A549 lung carcinoma cells. J. Biol. Chem. 272:33422-33429. - PubMed
-
- Carballo, E., W. S. Lai, and P. J. Blackshear. 1998. Feedback inhibition of macrophage tumor necrosis factor-α production by tristetraprolin. Science 281:1001-1005. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
