Primary immune responses by cord blood CD4(+) T cells and NK cells inhibit Epstein-Barr virus B-cell transformation in vitro
- PMID: 11967323
- PMCID: PMC136124
- DOI: 10.1128/jvi.76.10.5071-5081.2002
Primary immune responses by cord blood CD4(+) T cells and NK cells inhibit Epstein-Barr virus B-cell transformation in vitro
Erratum in
- J Virol 2002 Aug;76(16):8504
Abstract
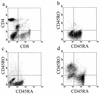
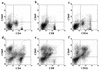
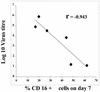
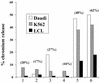
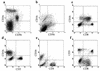
Epstein-Barr virus (EBV) transformation of B cells from fetal cord blood in vitro varies depending on the individual sample. When a single preparation of EBV was simultaneously used to transform fetal cord blood samples from six different individuals, the virus transformation titer varied from less than zero to 10(5.9). We show that this variation in EBV transformation is associated with a marked primary immune response in cord blood samples predominately involving CD4(+) T cells and CD16(+) CD56(+) NK cells. After virus challenge both CD4(+) T cells and NK cells in fetal cord blood cultures expressed the lymphocyte activation marker CD69. The cytotoxic response against autologous EBV-infected lymphoblastoid cell line (LCL) targets correlated with the number of CD16(+) CD69(+) cells and was inversely correlated with the virus transformation titer. Although NK activity was detected in fresh cord blood and increased following activation by the virus, killing of autologous LCLs was detected only following activation by exposure to the virus. Both activated CD4(+) T cells and CD16(+) NK cells were independently able to kill autologous LCLs. Both interleukin-2 and gamma interferon were produced by CD4(+) T cells after virus challenge. The titer of EBV was lower when purified B cells were used than when whole cord blood was used. Addition of monocytes restored the virus titer, while addition of resting T cells or EBV-activated CD4(+) T-cell blasts reduced the virus titer. We conclude that there are primary NK-cell and Th1-type CD4(+) T-cell responses to EBV in fetal cord blood that limit the expansion of EBV-infected cells and in some cases eliminate virus infection in vitro.
Figures

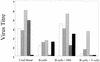
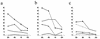
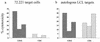

Similar articles
-
Primary CD4+ T-cell responses provide both helper and cytotoxic functions during Epstein-Barr virus infection and transformation of fetal cord blood B cells.J Virol. 2007 May;81(9):4766-75. doi: 10.1128/JVI.02608-06. Epub 2007 Feb 21. J Virol. 2007. PMID: 17314172 Free PMC article.
-
High frequency of Epstein-Barr virus (EBV) lymphoblastoid cell line-reactive lymphocytes in cord blood: evaluation of cytolytic activity and IL-2 production.Clin Exp Immunol. 1997 Feb;107(2):312-20. doi: 10.1111/j.1365-2249.1997.258-ce1131.x. Clin Exp Immunol. 1997. PMID: 9030869 Free PMC article.
-
Tonsilar NK cells restrict B cell transformation by the Epstein-Barr virus via IFN-gamma.PLoS Pathog. 2008 Feb 8;4(2):e27. doi: 10.1371/journal.ppat.0040027. PLoS Pathog. 2008. PMID: 18266470 Free PMC article.
-
Cellular responses to viral infection in humans: lessons from Epstein-Barr virus.Annu Rev Immunol. 2007;25:587-617. doi: 10.1146/annurev.immunol.25.022106.141553. Annu Rev Immunol. 2007. PMID: 17378764 Review.
-
Natural killer cell responses to human oncogenic γ-herpesvirus infections.Semin Immunol. 2022 Mar;60:101652. doi: 10.1016/j.smim.2022.101652. Epub 2022 Sep 23. Semin Immunol. 2022. PMID: 36162228 Review.
Cited by
-
Cytolytic CD4(+)-T-cell clones reactive to EBNA1 inhibit Epstein-Barr virus-induced B-cell proliferation.J Virol. 2003 Nov;77(22):12088-104. doi: 10.1128/jvi.77.22.12088-12104.2003. J Virol. 2003. PMID: 14581546 Free PMC article.
-
The interaction between PRRSV and the late gestation pig fetus.Virus Res. 2010 Dec;154(1-2):114-22. doi: 10.1016/j.virusres.2010.09.001. Epub 2010 Sep 9. Virus Res. 2010. PMID: 20832434 Free PMC article. Review.
-
Childcare attendance and risk of infectious mononucleosis: A population-based Danish cohort study.PLoS One. 2021 Dec 22;16(12):e0261665. doi: 10.1371/journal.pone.0261665. eCollection 2021. PLoS One. 2021. PMID: 34937060 Free PMC article.
-
Modeling Human Antitumor Responses In Vivo Using Umbilical Cord Blood-Engrafted Mice.Front Immunol. 2018 Jan 26;9:54. doi: 10.3389/fimmu.2018.00054. eCollection 2018. Front Immunol. 2018. PMID: 29434589 Free PMC article. Review.
-
Regression of Epstein-Barr virus-induced B-cell transformation in vitro involves virus-specific CD8+ T cells as the principal effectors and a novel CD4+ T-cell reactivity.J Virol. 2005 May;79(9):5477-88. doi: 10.1128/JVI.79.9.5477-5488.2005. J Virol. 2005. PMID: 15827162 Free PMC article.
References
-
- Ablashi, D., G. W. Bornkamm, C. Boshoff, S. H. Chan, I. Ernberg, I. T. Magrath, M. G. Masucci, M. Melbye, P. S. Moore, A. J. Morgan, N. Muller, G. Niedobitek, P. P. Pastoret, N. Raab-Traub, C. Rabkin, T. F. Schulz, G. de The, A. O. Williams, and M. C. Yu. 1997. Epstein-Barr virus and Kaposi's sarcoma herpesvirus/human herpesvirus. IARC Monogr. Eval. Carcinog. Risks Hum. 70:497.
-
- Andersson, J. 1998. Infectious mononucleosis: clinical characteristics, complications and management. Herpes 5:15-19.
-
- Atedzoe, B. N., A. Ahmad, and J. Menezes. 1997. Enhancement of natural killer cell cytotoxicity by the human herpesvirus-7 via IL-15 induction. J. Immunol. 159:4966-4972. - PubMed
-
- Avril, T., A. C. Jarousseau, H. Watier, J. Boucraut, P. Le Bouteiller, P. Bardos, and G. Thibault. 1999. Trophoblast cell line resistance to NK lysis mainly involves an HLA class I-independent mechanism. J. Immunol. 162:5902-5909. - PubMed
-
- Bamford, R. N., A. J. Grant, J. D. Burton, C. Peters, G. Kurys, C. K. Goldman, J. Brennan, E. Roessler, and T. A. Waldmann. 1994. The interleukin (IL) 2 receptor beta chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc. Natl. Acad. Sci. USA 91:4940-4944. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

