Human TATA binding protein inhibits human papillomavirus type 11 DNA replication by antagonizing E1-E2 protein complex formation on the viral origin of replication
- PMID: 11967317
- PMCID: PMC136168
- DOI: 10.1128/jvi.76.10.5014-5023.2002
Human TATA binding protein inhibits human papillomavirus type 11 DNA replication by antagonizing E1-E2 protein complex formation on the viral origin of replication
Abstract
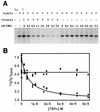
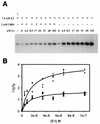
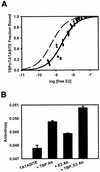
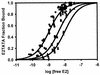
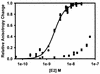
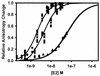
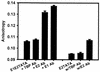
The human papillomavirus (HPV) protein E2 possesses dual roles in the viral life cycle. By interacting directly with host transcription factors in basal keratinocytes, E2 promotes viral transcription. As keratinocyte differentiation progresses, E2 associates with the viral helicase, E1, to activate vegetative viral DNA replication. How E2's major role switches from transcription to replication during keratinocyte differentiation is not understood, but the presence of a TATA site near the viral origin of replication led us to hypothesize that TATA-binding protein (TBP) could affect HPV replication. Here we show that the C-terminal domain of TBP (TBPc) is a potent inhibitor of E2-stimulated HPV DNA replication in vitro (50% inhibitory concentration = 0.56 nM). Increasing the E1 concentration could not overcome TBPc inhibition in replication assays, indicating that TBPc is a noncompetitive inhibitor of E1 binding. While direct E2-TBPc association could be demonstrated, this interaction could not fully account for the mechanism of TBPc-mediated inhibition of viral replication. Because E2 supports sequence-specific binding of E1 to the viral ori, we proposed that TBPc antagonizes E1-ori association indirectly through inhibition of E2-DNA binding. Indeed, TBPc potently antagonized E2 binding to DNA in the absence (K(i) = 0.5 +/- 0.1 nM) and presence (K(i) = 0.6 +/- 0.3 nM) of E1. Since E2 and TBPc cannot be coadjacent on viral sequences, direct DNA-binding competition between TBPc and E2 was responsible for replication inhibition. Given the ability of TBPc to inhibit HPV DNA replication in vitro and data indicating that TBPc antagonized E2-ori association, we propose that transcription factors regulate HPV DNA replication as well as viral transcription.
Figures

Similar articles
-
Identification of domains of the HPV11 E1 protein required for DNA replication in vitro.Virology. 2000 Jun 20;272(1):137-50. doi: 10.1006/viro.2000.0328. Virology. 2000. PMID: 10873756
-
DNA replication specificity and functional E2 interaction of the E1 proteins of human papillomavirus types 1a and 18 are determined by their carboxyl-terminal halves.Virology. 1999 Apr 10;256(2):330-9. doi: 10.1006/viro.1999.9665. Virology. 1999. PMID: 10191198
-
Interactions of the cellular CCAAT displacement protein and human papillomavirus E2 protein with the viral origin of replication can regulate DNA replication.Virology. 2006 Jul 5;350(2):302-11. doi: 10.1016/j.virol.2006.01.047. Epub 2006 Mar 9. Virology. 2006. PMID: 16529788
-
Control of viral replication and transcription by the papillomavirus E8^E2 protein.Virus Res. 2017 Mar 2;231:96-102. doi: 10.1016/j.virusres.2016.11.005. Epub 2016 Nov 4. Virus Res. 2017. PMID: 27825778 Review.
-
Papillomavirus E1 proteins: form, function, and features.Virus Genes. 2002 Jun;24(3):275-90. doi: 10.1023/a:1015336817836. Virus Genes. 2002. PMID: 12086149 Review.
Cited by
-
Regulation of the life cycle of HPVs by differentiation and the DNA damage response.Future Microbiol. 2013 Dec;8(12):1547-57. doi: 10.2217/fmb.13.127. Future Microbiol. 2013. PMID: 24266355 Free PMC article. Review.
-
Productive Lifecycle of Human Papillomaviruses that Depends Upon Squamous Epithelial Differentiation.Front Microbiol. 2012 Apr 24;3:152. doi: 10.3389/fmicb.2012.00152. eCollection 2012. Front Microbiol. 2012. PMID: 22536200 Free PMC article.
-
DNA damage response is hijacked by human papillomaviruses to complete their life cycle.J Zhejiang Univ Sci B. 2017 Mar;18(3):215-232. doi: 10.1631/jzus.B1600306. J Zhejiang Univ Sci B. 2017. PMID: 28271657 Free PMC article. Review.
-
Functional mapping of the human papillomavirus type 16 E1 cistron.J Virol. 2008 Nov;82(21):10724-34. doi: 10.1128/JVI.00921-08. Epub 2008 Aug 27. J Virol. 2008. PMID: 18753208 Free PMC article.
-
Identification of peptides that inhibit the DNA binding, trans-activator, and DNA replication functions of the human papillomavirus type 11 E2 protein.J Virol. 2004 Mar;78(5):2637-41. doi: 10.1128/jvi.78.5.2637-2641.2003. J Virol. 2004. PMID: 14963172 Free PMC article.
References
-
- Apt, D., R. M. Watts, G. Suske, and H. U. Bernard. 1996. High Sp1/Sp3 ratios in epithelial cells during epithelial differentiation and cellular transformation correlate with the activation of the HPV-16 promoter. Virology 224:281-291. - PubMed
-
- Chao, S.-F., W. J. Rocque, S. Daniel, L. E. Czyzyk, W. C. Phelps, and K. A. Alexander. 1999. Subunit affinities and stoichiometries of the human papillomavirus type 11 E1:E2:DNA complex. Biochemistry 38:4586-4594. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

