A Los1p-independent pathway for nuclear export of intronless tRNAs in Saccharomycescerevisiae
- PMID: 11959996
- PMCID: PMC122783
- DOI: 10.1073/pnas.082682699
A Los1p-independent pathway for nuclear export of intronless tRNAs in Saccharomycescerevisiae
Abstract
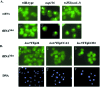
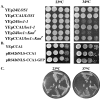
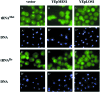
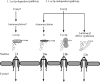
Los1p, the Saccharomyces cerevisiae exportin-t homologue, binds tRNA and functions in pre-tRNA splicing and export of mature tRNA from the nucleus to the cytosol. Because LOS1 is unessential in yeast, other pathways for tRNA nuclear export must exist. We report that Cca1p, which adds nucleotides C, C, and A to the 3' end of tRNAs, is a multicopy suppressor of the defect in tRNA nuclear export caused by los1 null mutations. Mes1p, methionyl-tRNA synthetase, also suppresses the defect in nuclear export of tRNA(Met) in los1 cells. Thus, Cca1p and Mes1p seem to function in a Los1p-independent tRNA nuclear export pathway. Heterokaryon analysis indicates that Cca1p is a nucleus/cytosol-shuttling protein, providing the potential for Cca1p to function as an exporter or an adapter in this tRNA nuclear export pathway. In yeast, most mutations that affect tRNA nuclear export also cause defects in pre-tRNA splicing leading to tight coupling of the splicing and export processes. In contrast, we show that overexpressed Cca1p corrects the nuclear export, but not the pre-tRNA-splicing defects of los1Kan(r) cells, thereby uncoupling pre-tRNA splicing and tRNA nuclear export.
Figures


Similar articles
-
The nuclear tRNA aminoacylation-dependent pathway may be the principal route used to export tRNA from the nucleus in Saccharomyces cerevisiae.Biochem J. 2004 Mar 15;378(Pt 3):809-16. doi: 10.1042/BJ20031306. Biochem J. 2004. PMID: 14640976 Free PMC article.
-
tRNA nuclear export in saccharomyces cerevisiae: in situ hybridization analysis.Mol Biol Cell. 1998 Nov;9(11):3041-55. doi: 10.1091/mbc.9.11.3041. Mol Biol Cell. 1998. PMID: 9802895 Free PMC article.
-
Role of nuclear pools of aminoacyl-tRNA synthetases in tRNA nuclear export.Mol Biol Cell. 2001 May;12(5):1381-92. doi: 10.1091/mbc.12.5.1381. Mol Biol Cell. 2001. PMID: 11359929 Free PMC article.
-
Review: transport of tRNA out of the nucleus-direct channeling to the ribosome?J Struct Biol. 2000 Apr;129(2-3):288-94. doi: 10.1006/jsbi.2000.4226. J Struct Biol. 2000. PMID: 10806079 Review.
-
The ins and outs of nuclear re-export of retrogradely transported tRNAs in Saccharomyces cerevisiae.Nucleus. 2010 May-Jun;1(3):224-30. doi: 10.4161/nucl.1.3.11250. Epub 2010 Jan 13. Nucleus. 2010. PMID: 21327067 Free PMC article. Review.
Cited by
-
Cellular dynamics of tRNAs and their genes.FEBS Lett. 2010 Jan 21;584(2):310-7. doi: 10.1016/j.febslet.2009.11.053. FEBS Lett. 2010. PMID: 19931532 Free PMC article. Review.
-
Transfer RNA travels from the cytoplasm to organelles.Wiley Interdiscip Rev RNA. 2011 Nov-Dec;2(6):802-17. doi: 10.1002/wrna.93. Epub 2011 Jul 11. Wiley Interdiscip Rev RNA. 2011. PMID: 21976284 Free PMC article. Review.
-
Rsp5 ubiquitin ligase modulates translation accuracy in yeast Saccharomyces cerevisiae.RNA. 2005 Nov;11(11):1710-8. doi: 10.1261/rna.2131605. Epub 2005 Sep 21. RNA. 2005. PMID: 16177134 Free PMC article.
-
Genome-wide investigation of the role of the tRNA nuclear-cytoplasmic trafficking pathway in regulation of the yeast Saccharomyces cerevisiae transcriptome and proteome.Mol Cell Biol. 2013 Nov;33(21):4241-54. doi: 10.1128/MCB.00785-13. Epub 2013 Aug 26. Mol Cell Biol. 2013. PMID: 23979602 Free PMC article.
-
Maf1-mediated regulation of yeast RNA polymerase III is correlated with CCA addition at the 3' end of tRNA precursors.Gene. 2017 May 15;612:12-18. doi: 10.1016/j.gene.2016.08.033. Epub 2016 Aug 27. Gene. 2017. PMID: 27575455 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

