The LIM-homeodomain transcription factor Lmx1b plays a crucial role in podocytes
- PMID: 11956245
- PMCID: PMC150943
- DOI: 10.1172/JCI13961
The LIM-homeodomain transcription factor Lmx1b plays a crucial role in podocytes
Abstract
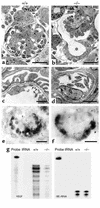
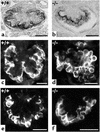
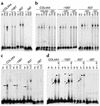
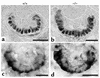
Patients with nail-patella syndrome often suffer from a nephropathy, which ultimately results in chronic renal failure. The finding that this disease is caused by mutations in the transcription factor LMX1B, which in the kidney is expressed exclusively in podocytes, offers the opportunity for a better understanding of the renal pathogenesis. In our analysis of the nephropathy in nail-patella syndrome, we have made use of the Lmx1b knockout mouse. Transmission electron micrographs showed that glomerular development in general and the differentiation of podocytes in particular were severely impaired. The glomerular capillary network was poorly elaborated, fenestrae in the endothelial cells were largely missing, and the glomerular basement membrane was split. In addition podocytes retained a cuboidal shape and did not form foot processes and slit diaphragms. Expression of the alpha4 chain of collagen IV and of podocin was also severely reduced. Using gel shift assays, we demonstrated that LMX1B bound to two AT-rich sequences in the promoter region of NPHS2, the gene encoding podocin. Our results demonstrate that Lmx1b regulates important steps in glomerular development and establish a link between three hereditary kidney diseases: nail-patella syndrome (Lmx1b), steroid-resistant nephrotic syndrome (podocin), and Alport syndrome (collagen IV alpha4).
Figures



Similar articles
-
In vivo expression of putative LMX1B targets in nail-patella syndrome kidneys.Am J Pathol. 2003 Jul;163(1):145-55. doi: 10.1016/S0002-9440(10)63638-3. Am J Pathol. 2003. PMID: 12819019 Free PMC article.
-
Regulation of glomerular basement membrane collagen expression by LMX1B contributes to renal disease in nail patella syndrome.Nat Genet. 2001 Feb;27(2):205-8. doi: 10.1038/84853. Nat Genet. 2001. PMID: 11175791
-
Transcriptional induction of slit diaphragm genes by Lmx1b is required in podocyte differentiation.J Clin Invest. 2002 Apr;109(8):1065-72. doi: 10.1172/JCI13954. J Clin Invest. 2002. PMID: 11956244 Free PMC article.
-
Nail-patella syndrome. Overview on clinical and molecular findings.Pediatr Nephrol. 2002 Sep;17(9):703-12. doi: 10.1007/s00467-002-0911-5. Epub 2002 Jul 30. Pediatr Nephrol. 2002. PMID: 12215822 Review.
-
[From gene to disease; the nail-patella syndrome and the LMX1B gene].Ned Tijdschr Geneeskd. 2003 Jan 11;147(2):67-9. Ned Tijdschr Geneeskd. 2003. PMID: 12602071 Review. Dutch.
Cited by
-
A novel small deletion of LMX1B in a large Chinese family with nail-patella syndrome.BMC Med Genet. 2019 May 3;20(1):71. doi: 10.1186/s12881-019-0801-3. BMC Med Genet. 2019. PMID: 31053111 Free PMC article.
-
Dysregulation of WTI (-KTS) is Associated with the Kidney-Specific Effects of the LMX1B R246Q Mutation.Sci Rep. 2017 Jan 6;7:39933. doi: 10.1038/srep39933. Sci Rep. 2017. PMID: 28059119 Free PMC article.
-
Notch signaling, wt1 and foxc2 are key regulators of the podocyte gene regulatory network in Xenopus.Development. 2010 Jun;137(11):1863-73. doi: 10.1242/dev.042887. Epub 2010 Apr 28. Development. 2010. PMID: 20431116 Free PMC article.
-
Semaphorin3a regulates endothelial cell number and podocyte differentiation during glomerular development.Development. 2009 Dec;136(23):3979-89. doi: 10.1242/dev.037267. Development. 2009. PMID: 19906865 Free PMC article.
-
LIM-homeodomain genes in mammalian development and human disease.Mol Biol Rep. 2005 Jun;32(2):67-77. doi: 10.1007/s11033-004-7657-z. Mol Biol Rep. 2005. PMID: 16022279 Review.
References
-
- Bennett WM, et al. The nephropathy of the nail-patella syndrome. Clinicopathologic analysis of 11 kindred. Am J Med. 1973;54:304–319. - PubMed
-
- del Pozo E, Lapp H. Ultrastructure of the kidney in the nephropathy of the nail-patella syndrome. Am J Clin Pathol. 1970;54:845–851. - PubMed
-
- Ben-Bassat M, Cohen L, Rosenfeld J. The glomerular basement membrane in the nail-patella syndrome. Arch Pathol. 1971;92:350–355. - PubMed
-
- Drut RM, Chandra S, Latorraca R, Gilbert-Barness E. Nail-patella syndrome in a spontaneously aborted 18-week fetus: ultrastructural and immunofluorescent study of the kidneys. Am J Med Genet. 1992;43:693–696. - PubMed
-
- Lubec B, Arbeiter K, Ulrich W, Frauscher G. Hereditary osteo-onycho-renal dysplasia with excess urinary pyridinoline cross-links and abnormal kidney collagen cross-linking. Nephron. 1995;70:255–259. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

