A novel mechanism of response selectivity of neurons in cat visual cortex
- PMID: 11927689
- PMCID: PMC2290213
- DOI: 10.1113/jphysiol.2001.012974
A novel mechanism of response selectivity of neurons in cat visual cortex
Abstract
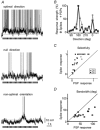
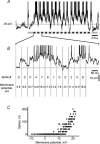
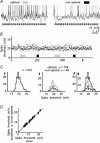
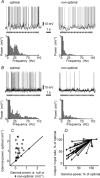
The spiking of cortical neurons critically depends on properties of the afferent stimuli. In the visual cortex, neurons respond selectively to the orientation and direction of movement of an object. The orientation and direction selectivity is improved upon transformation of the membrane potential changes into trains of action potentials. To address the question of whether the transformation of the membrane potential changes into spiking of a cell depends on the stimulus orientation and the direction of movement, we made intracellular recordings from the cat visual cortex in vivo during presentation of moving gratings of different orientations. We found that the relationship between the membrane polarization and the firing rate (input-output transfer function) depended on the stimulus orientation. The input-output transfer function was steepest during responses to the optimal stimulus; membrane depolarization of a given amplitude led to generation of more action potentials when evoked by an optimal stimulus than during non-optimal stimulation. The threshold for the action potential generation did not depend on stimulus orientation, and thus could not account for the observed difference in the transfer function. Oscillations of the membrane potential in the gamma-frequency range (25-70 Hz) were most pronounced during optimal stimulation and their strength changed in parallel with the changes in the transfer function, suggesting a possible relationship between the two parameters. We suggest that the improved input-output relationship of neurons during optimal stimulation represents a novel mechanism that may contribute to the final sharp orientation selectivity of spike responses in the cortical cells.
Figures

Similar articles
-
Comparison of the selectivity of postsynaptic potentials and spike responses in cat visual cortex.Eur J Neurosci. 2000 Jan;12(1):257-63. doi: 10.1046/j.1460-9568.2000.00909.x. Eur J Neurosci. 2000. PMID: 10651880
-
Direction selectivity of synaptic potentials in simple cells of the cat visual cortex.J Neurophysiol. 1997 Nov;78(5):2772-89. doi: 10.1152/jn.1997.78.5.2772. J Neurophysiol. 1997. PMID: 9356425
-
Orientation selectivity of synaptic input to neurons in mouse and cat primary visual cortex.J Neurosci. 2011 Aug 24;31(34):12339-50. doi: 10.1523/JNEUROSCI.2039-11.2011. J Neurosci. 2011. PMID: 21865476 Free PMC article.
-
Non-linear dynamics of columns of cat visual cortex revealed by simulation and experiment.Ciba Found Symp. 1994;184:88-99; discussion 99-103, 120-8. doi: 10.1002/9780470514610.ch5. Ciba Found Symp. 1994. PMID: 7882763 Review.
-
Recognizing the visual stimulus from neuronal discharges.Trends Neurosci. 1991 Jul;14(7):282-6. doi: 10.1016/0166-2236(91)90138-k. Trends Neurosci. 1991. PMID: 1719674 Review.
Cited by
-
Stimulus-dependent changes in spike threshold enhance feature selectivity in rat barrel cortex neurons.J Neurosci. 2005 Mar 16;25(11):2983-91. doi: 10.1523/JNEUROSCI.4906-04.2005. J Neurosci. 2005. PMID: 15772358 Free PMC article.
-
Neuronal integration of synaptic input in the fluctuation-driven regime.J Neurosci. 2004 Mar 10;24(10):2345-56. doi: 10.1523/JNEUROSCI.3349-03.2004. J Neurosci. 2004. PMID: 15014109 Free PMC article.
-
Short-term depression in thalamocortical synapses of cat primary visual cortex.J Neurosci. 2005 Aug 3;25(31):7179-90. doi: 10.1523/JNEUROSCI.1445-05.2005. J Neurosci. 2005. PMID: 16079400 Free PMC article.
-
Amplification of trial-to-trial response variability by neurons in visual cortex.PLoS Biol. 2004 Sep;2(9):E264. doi: 10.1371/journal.pbio.0020264. Epub 2004 Aug 24. PLoS Biol. 2004. PMID: 15328535 Free PMC article.
-
Spatial and temporal features of synaptic to discharge receptive field transformation in cat area 17.J Neurophysiol. 2010 Feb;103(2):677-97. doi: 10.1152/jn.90946.2008. Epub 2009 Nov 11. J Neurophysiol. 2010. PMID: 19906874 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

