Activation and caspase-mediated inhibition of PARP: a molecular switch between fibroblast necrosis and apoptosis in death receptor signaling
- PMID: 11907276
- PMCID: PMC99613
- DOI: 10.1091/mbc.01-05-0272
Activation and caspase-mediated inhibition of PARP: a molecular switch between fibroblast necrosis and apoptosis in death receptor signaling
Abstract
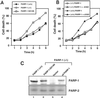
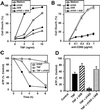
Death ligands not only induce apoptosis but can also trigger necrosis with distinct biochemical and morphological features. We recently showed that in L929 cells CD95 ligation induces apoptosis, whereas TNF elicits necrosis. Treatment with anti-CD95 resulted in typical apoptosis characterized by caspase activation and DNA fragmentation. These events were barely induced by TNF, although TNF triggered cell death to a similar extent as CD95. Surprisingly, whereas the caspase inhibitor zVAD prevented CD95-mediated apoptosis, it potentiated TNF-induced necrosis. Cotreatment with TNF and zVAD was characterized by ATP depletion and accelerated necrosis. To investigate the mechanisms underlying TNF-induced cell death and its potentiation by zVAD, we examined the role of poly(ADP-ribose)polymerase-1 (PARP-1). TNF but not CD95 mediated PARP activation, whereas a PARP inhibitor suppressed TNF-induced necrosis and the sensitizing effect of zVAD. In addition, fibroblasts expressing a noncleavable PARP-1 mutant were more sensitive to TNF than wild-type cells. Our results indicate that TNF induces PARP activation leading to ATP depletion and subsequent necrosis. In contrast, in CD95-mediated apoptosis caspases cause PARP-1 cleavage and thereby maintain ATP levels. Because ATP is required for apoptosis, we suggest that PARP-1 cleavage functions as a molecular switch between apoptotic and necrotic modes of death receptor-induced cell death.
Figures




Similar articles
-
Caspase inhibition in apoptotic T cells triggers necrotic cell death depending on the cell type and the proapoptotic stimulus.J Cell Biochem. 2006 Apr 15;97(6):1350-61. doi: 10.1002/jcb.20670. J Cell Biochem. 2006. PMID: 16365881
-
Caspase inhibition switches the mode of cell death induced by cyanide by enhancing reactive oxygen species generation and PARP-1 activation.Toxicol Appl Pharmacol. 2004 Mar 1;195(2):194-202. doi: 10.1016/j.taap.2003.11.012. Toxicol Appl Pharmacol. 2004. PMID: 14998685
-
A serine protease is involved in the initiation of DNA damage-induced apoptosis.Cell Death Differ. 2003 Oct;10(10):1204-12. doi: 10.1038/sj.cdd.4401296. Cell Death Differ. 2003. PMID: 14502243
-
Caspase inhibitors promote alternative cell death pathways.Sci STKE. 2006 Oct 24;2006(358):pe44. doi: 10.1126/stke.3582006pe44. Sci STKE. 2006. PMID: 17062895 Review.
-
Poly(ADP-ribose) polymerase-1 mediated caspase-independent cell death after ischemia/reperfusion.Free Radic Biol Med. 2005 Jul 1;39(1):81-90. doi: 10.1016/j.freeradbiomed.2005.03.021. Epub 2005 Apr 8. Free Radic Biol Med. 2005. PMID: 15925280 Review.
Cited by
-
Opposite Expression of SPARC between the Liver and Pancreas in Streptozotocin-Induced Diabetic Rats.PLoS One. 2015 Jun 25;10(6):e0131189. doi: 10.1371/journal.pone.0131189. eCollection 2015. PLoS One. 2015. PMID: 26110898 Free PMC article.
-
A Review on Annona muricata and Its Anticancer Activity.Cancers (Basel). 2022 Sep 19;14(18):4539. doi: 10.3390/cancers14184539. Cancers (Basel). 2022. PMID: 36139697 Free PMC article. Review.
-
8-Hydroxydaidzein Induces Apoptosis and Inhibits AML-Associated Gene Expression in U-937 Cells: Potential Phytochemical for AML Treatment.Biomolecules. 2023 Oct 26;13(11):1575. doi: 10.3390/biom13111575. Biomolecules. 2023. PMID: 38002257 Free PMC article.
-
The Effect of Biotinylated PAMAM G3 Dendrimers Conjugated with COX-2 Inhibitor (celecoxib) and PPARγ Agonist (Fmoc-L-Leucine) on Human Normal Fibroblasts, Immortalized Keratinocytes and Glioma Cells in Vitro.Molecules. 2019 Oct 22;24(20):3801. doi: 10.3390/molecules24203801. Molecules. 2019. PMID: 31652556 Free PMC article.
-
Nitric oxide and peroxynitrite in health and disease.Physiol Rev. 2007 Jan;87(1):315-424. doi: 10.1152/physrev.00029.2006. Physiol Rev. 2007. PMID: 17237348 Free PMC article. Review.
References
-
- Ame JC, Rolli V, Schreiber V, Niedergang C, Apiou F, Decker P, Muller S, Hoger T, Menissier-de Murcia J, de Murcia G. PARP-2, a novel mammalian DNA damage-dependent poly(ADP-ribose) polymerase. J Biol Chem. 1999;274:17860–17868. - PubMed
-
- Avila MA, Velasco JA, Smulson ME, Dritschilo A, Castro R, Notario V. Functional expression of human poly(ADP-ribose) polymerase in Schizosaccharomyces pombe results in mitotic delay at G1, increased mutation rate, and sensitization to radiation. Yeast. 1994;10:1003–1017. - PubMed
-
- Bürkle A. Physiology and pathophysiology of poly(ADP-ribosyl)ation. Bioessays. 2001;9:795–806. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

