Progressive cardiac hypertrophy and dysfunction in atrial natriuretic peptide receptor (GC-A) deficient mice
- PMID: 11907014
- PMCID: PMC1767056
- DOI: 10.1136/heart.87.4.368
Progressive cardiac hypertrophy and dysfunction in atrial natriuretic peptide receptor (GC-A) deficient mice
Abstract
Objective: To investigate how permanent inhibition of guanylyl cyclase A receptor (GC-A) affects cardiac function.
Methods: Hearts of GC-A-/- and corresponding wild type mice (GC-A+/+) were characterised by histological, western blotting, and northern blotting analyses. Cardiac function was evaluated in isolated, working heart preparations.
Results: At 4 months of age, GC-A-/- mice had global cardiac hypertrophy (about a 40% increase in cardiac weight) without interstitial fibrosis. Examination of heart function found a significant delay in the time of relaxation; all other parameters of cardiac contractility were similar to those in wild type mice. At 12 months, the hypertrophic changes were much more severe (about a 61% increase in cardiac weight), together with a shift in cardiac gene expression (enhanced concentrations of atrial natriuretic peptide (3.8-fold), B type natriuretic peptide (2-fold), beta myosin heavy chain (1.6-fold) and alpha skeletal actin (1.7-fold) mRNA), increased expression of cytoskeletal tubulin and desmin (by 29.6% and 25.6%, respectively), and pronounced interstitial fibrosis. These changes were associated with significantly impaired cardiac contractility (+dP/dt decreased by about 10%) and relaxation (-dP/dt decreased by 21%), as well as depressed contractile responses to pressure load (all p < 0.05).
Conclusions: Chronic hypertension in GC-A-/- mice is associated with progressive cardiac changes--namely, initially compensated cardiomyocyte hypertrophy, which is complicated by interstitial fibrosis and impaired cardiac contractility at later stages.
Figures
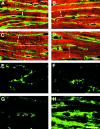

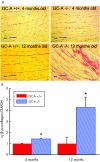

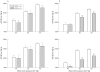
Comment in
-
Natriuretic peptide receptors and the heart.Heart. 2002 Apr;87(4):314-5. doi: 10.1136/heart.87.4.314. Heart. 2002. PMID: 11906995 Free PMC article. No abstract available.
Similar articles
-
Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A.J Clin Invest. 2003 May;111(9):1399-407. doi: 10.1172/JCI17061. J Clin Invest. 2003. PMID: 12727932 Free PMC article.
-
Calcineurin-nuclear factor of activated T cells pathway-dependent cardiac remodeling in mice deficient in guanylyl cyclase A, a receptor for atrial and brain natriuretic peptides.Circulation. 2005 Jun 14;111(23):3095-104. doi: 10.1161/CIRCULATIONAHA.104.510594. Epub 2005 Jun 6. Circulation. 2005. PMID: 15939815
-
Involvement of the NF-kappa B/matrix metalloproteinase pathway in cardiac fibrosis of mice lacking guanylyl cyclase/natriuretic peptide receptor A.J Biol Chem. 2005 May 13;280(19):19230-42. doi: 10.1074/jbc.M411373200. Epub 2005 Feb 13. J Biol Chem. 2005. PMID: 15710627
-
CaMKII-mediated increased lusitropic responses to beta-adrenoreceptor stimulation in ANP-receptor deficient mice.Cardiovasc Res. 2007 Mar 1;73(4):678-88. doi: 10.1016/j.cardiores.2006.10.003. Epub 2006 Oct 7. Cardiovasc Res. 2007. PMID: 17107670
-
C-type natriuretic peptide and guanylyl cyclase B receptor.Peptides. 2005 Jun;26(6):1024-34. doi: 10.1016/j.peptides.2004.08.027. Epub 2005 Apr 15. Peptides. 2005. PMID: 15911070 Review.
Cited by
-
Systemic, but not cardiomyocyte-specific, deletion of the natriuretic peptide receptor guanylyl cyclase A increases cardiomyocyte number in neonatal mice.Histochem Cell Biol. 2015 Oct;144(4):365-75. doi: 10.1007/s00418-015-1337-z. Epub 2015 Jun 10. Histochem Cell Biol. 2015. PMID: 26059418
-
Increased atherosclerosis and smooth muscle cell hypertrophy in natriuretic peptide receptor A-/-apolipoprotein E-/- mice.Arterioscler Thromb Vasc Biol. 2003 Jun 1;23(6):1077-82. doi: 10.1161/01.ATV.0000071702.45741.2E. Epub 2003 Apr 17. Arterioscler Thromb Vasc Biol. 2003. PMID: 12702516 Free PMC article.
-
A friend within the heart: natriuretic peptide receptor signaling.J Clin Invest. 2003 May;111(9):1275-7. doi: 10.1172/JCI18389. J Clin Invest. 2003. PMID: 12727915 Free PMC article. No abstract available.
-
B Type natriuretic peptide: a good omen in myocardial ischaemia?Heart. 2003 Jul;89(7):707-9. doi: 10.1136/heart.89.7.707. Heart. 2003. PMID: 12807835 Free PMC article. No abstract available.
-
Natriuretic peptide receptor B signaling in the cardiovascular system: protection from cardiac hypertrophy.J Mol Med (Berl). 2007 Aug;85(8):797-810. doi: 10.1007/s00109-007-0183-4. Epub 2007 Apr 12. J Mol Med (Berl). 2007. PMID: 17429599 Review.
References
-
- Anand-Srivastava MB, Trachte GJ. Atrial natriuretic factor receptors and signal transduction mechanisms. Pharmacol Rev 1993;45:455–97. - PubMed
-
- Lopez MJ, Wong SK, Kishimoto I, et al. Salt-resistant hypertension in mice lacking the guanylyl cyclase-A receptor for atrial natriuretic peptide. Nature 1995;378:65–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
