Neuregulin expression at neuromuscular synapses is modulated by synaptic activity and neurotrophic factors
- PMID: 11896160
- PMCID: PMC6758272
- DOI: 10.1523/JNEUROSCI.22-06-02206.2002
Neuregulin expression at neuromuscular synapses is modulated by synaptic activity and neurotrophic factors
Abstract
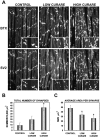
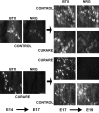
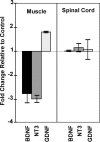
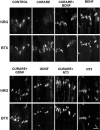
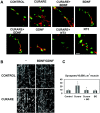
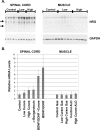
The proper formation of neuromuscular synapses requires ongoing synaptic activity that is translated into complex structural changes to produce functional synapses. One mechanism by which activity could be converted into these structural changes is through the regulated expression of specific synaptic regulatory factors. Here we demonstrate that blocking synaptic activity with curare reduces synaptic neuregulin expression in a dose-dependent manner yet has little effect on synaptic agrin or a muscle-derived heparan sulfate proteoglycan. These changes are associated with a fourfold increase in number and a twofold reduction in average size of synaptic acetylcholine receptor clusters that appears to be caused by excessive axonal sprouting with the formation of new, smaller acetylcholine receptor clusters. Activity blockade also leads to threefold reductions in brain-derived neurotrophic factor and neurotrophin 3 expression in muscle without appreciably changing the expression of these same factors in spinal cord. Adding back these or other neurotrophic factors restores synaptic neuregulin expression and maintains normal end plate band architecture in the presence of activity blockade. The expression of neuregulin protein at synapses is independent of spinal cord and muscle neuregulin mRNA levels, suggesting that neuregulin accumulation at synapses is independent of transcription. These findings suggest a local, positive feedback loop between synaptic regulatory factors that translates activity into structural changes at neuromuscular synapses.
Figures

Similar articles
-
Neurotrophic factors increase neuregulin expression in embryonic ventral spinal cord neurons.J Neurosci. 1997 Feb 15;17(4):1416-24. doi: 10.1523/JNEUROSCI.17-04-01416.1997. J Neurosci. 1997. PMID: 9006983 Free PMC article.
-
Neuregulin: an activity-dependent synaptic modulator at the neuromuscular junction.J Neurocytol. 2003 Jun-Sep;32(5-8):649-64. doi: 10.1023/B:NEUR.0000020640.84708.35. J Neurocytol. 2003. PMID: 15034258 Review.
-
Synergistic effects of neuregulin and agrin on muscle acetylcholine receptor expression.Mol Cell Neurosci. 2004 Aug;26(4):558-69. doi: 10.1016/j.mcn.2004.04.009. Mol Cell Neurosci. 2004. PMID: 15276157
-
Regulation of quantal secretion by neurotrophic factors at developing motoneurons in Xenopus cell cultures.J Physiol. 1997 Aug 15;503 ( Pt 1)(Pt 1):129-39. doi: 10.1111/j.1469-7793.1997.129bi.x. J Physiol. 1997. PMID: 9288681 Free PMC article.
-
Modulation of hippocampal synaptic transmission and plasticity by neurotrophins.Prog Brain Res. 2000;128:231-41. doi: 10.1016/S0079-6123(00)28020-5. Prog Brain Res. 2000. PMID: 11105682 Review. No abstract available.
Cited by
-
Complexity of trophic factor signaling in experimental autoimmune encephalomyelitis: differential expression of neurotrophic and gliotrophic factors.J Neuroimmunol. 2013 Sep 15;262(1-2):11-8. doi: 10.1016/j.jneuroim.2013.05.012. Epub 2013 Jun 12. J Neuroimmunol. 2013. PMID: 23763772 Free PMC article.
-
Age-associated alterations of the neuromuscular junction.Exp Gerontol. 2011 Feb-Mar;46(2-3):193-8. doi: 10.1016/j.exger.2010.08.029. Epub 2010 Sep 18. Exp Gerontol. 2011. PMID: 20854887 Free PMC article. Review.
-
Extrasynaptic alpha 7-nicotinic acetylcholine receptor expression in developing neurons is regulated by inputs, targets, and activity.J Neurosci. 2002 Sep 15;22(18):8101-9. doi: 10.1523/JNEUROSCI.22-18-08101.2002. J Neurosci. 2002. PMID: 12223564 Free PMC article.
-
A novel BDNF gene promoter directs expression to skeletal muscle.BMC Neurosci. 2003 Jun 18;4:11. doi: 10.1186/1471-2202-4-11. BMC Neurosci. 2003. PMID: 12812528 Free PMC article.
-
Neuromuscular adaptations to respiratory muscle inactivity.Respir Physiol Neurobiol. 2009 Nov 30;169(2):133-40. doi: 10.1016/j.resp.2009.09.002. Epub 2009 Sep 8. Respir Physiol Neurobiol. 2009. PMID: 19744580 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
