The protein encoded by oncogene 6b from Agrobacterium tumefaciens interacts with a nuclear protein of tobacco
- PMID: 11884686
- PMCID: PMC152924
- DOI: 10.1105/tpc.010360
The protein encoded by oncogene 6b from Agrobacterium tumefaciens interacts with a nuclear protein of tobacco
Abstract
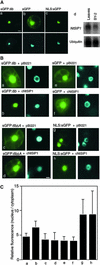
The 6b gene in the T-DNA from Agrobacterium has oncogenic activity in plant cells, inducing tumor formation, the phytohormone-independent division of cells, and alterations in leaf morphology. The product of the 6b gene appears to promote some aspects of the proliferation of plant cells, but the molecular mechanism of its action remains unknown. We report here that the 6b protein associates with a nuclear protein in tobacco that we have designated NtSIP1 (for Nicotiana tabacum 6b-interacting protein 1). NtSIP1 appears to be a transcription factor because its predicted amino acid sequence includes two regions that resemble a nuclear localization signal and a putative DNA binding motif, which is similar in terms of amino acid sequence to the triple helix motif of rice transcription factor GT-2. Expression in tobacco cells of a fusion protein composed of the DNA binding domain of the yeast GAL4 protein and the 6b protein activated the transcription of a reporter gene that was under the control of a chimeric promoter that included the GAL4 upstream activating sequence and the 35S minimal promoter of Cauliflower mosaic virus. Furthermore, nuclear localization of green fluorescent protein-fused 6b protein was enhanced by NtSIP1. A cluster of acidic residues in the 6b protein appeared to be essential for nuclear localization and for transactivation as well as for the hormone-independent growth of tobacco cells. Thus, it seems possible that the 6b protein might function in the proliferation of plant cells, at least in part, through an association with NtSIP1.
Figures



Similar articles
-
Interaction between Agrobacterium tumefaciens oncoprotein 6b and a tobacco nucleolar protein that is homologous to TNP1 encoded by a transposable element of Antirrhinum majus.J Plant Res. 2008 Jul;121(4):425-33. doi: 10.1007/s10265-008-0160-1. Epub 2008 May 8. J Plant Res. 2008. PMID: 18463947
-
Interaction between the tobacco DNA-binding activity CBF and the cyt-1 promoter element of the Agrobacterium tumefaciens T-DNA gene T-CYT correlates with cyt-1 directed gene expression in multiple tobacco tissue types.Plant J. 1993 Sep;4(3):525-34. doi: 10.1046/j.1365-313x.1993.04030525.x. Plant J. 1993. PMID: 8220494
-
The ethylene-responsive factor like protein 1 (CaERFLP1) of hot pepper (Capsicum annuum L.) interacts in vitro with both GCC and DRE/CRT sequences with different binding affinities: possible biological roles of CaERFLP1 in response to pathogen infection and high salinity conditions in transgenic tobacco plants.Plant Mol Biol. 2004 May;55(1):61-81. doi: 10.1007/s11103-004-0417-6. Plant Mol Biol. 2004. PMID: 15604665
-
Protein encoded by oncogene 6b from Agrobacterium tumefaciens has a reprogramming potential and histone chaperone-like activity.Front Plant Sci. 2014 Oct 28;5:572. doi: 10.3389/fpls.2014.00572. eCollection 2014. Front Plant Sci. 2014. PMID: 25389429 Free PMC article. Review.
-
SWIM, a novel Zn-chelating domain present in bacteria, archaea and eukaryotes.Trends Biochem Sci. 2002 Aug;27(8):384-6. doi: 10.1016/s0968-0004(02)02140-0. Trends Biochem Sci. 2002. PMID: 12151216 Review.
Cited by
-
Inhibition of Arabidopsis thaliana CIN-like TCP transcription factors by Agrobacterium T-DNA-encoded 6B proteins.Plant J. 2020 Mar;101(6):1303-1317. doi: 10.1111/tpj.14591. Epub 2019 Dec 5. Plant J. 2020. PMID: 31659801 Free PMC article.
-
The VirD2 pilot protein of Agrobacterium-transferred DNA interacts with the TATA box-binding protein and a nuclear protein kinase in plants.Proc Natl Acad Sci U S A. 2003 Aug 19;100(17):10108-13. doi: 10.1073/pnas.1733208100. Epub 2003 Aug 4. Proc Natl Acad Sci U S A. 2003. PMID: 12900506 Free PMC article.
-
Repression of seed maturation genes by a trihelix transcriptional repressor in Arabidopsis seedlings.Plant Cell. 2009 Jan;21(1):54-71. doi: 10.1105/tpc.108.061309. Epub 2009 Jan 20. Plant Cell. 2009. PMID: 19155348 Free PMC article.
-
Interaction of Arabidopsis Trihelix-Domain Transcription Factors VFP3 and VFP5 with Agrobacterium Virulence Protein VirF.PLoS One. 2015 Nov 16;10(11):e0142128. doi: 10.1371/journal.pone.0142128. eCollection 2015. PLoS One. 2015. PMID: 26571494 Free PMC article.
-
Morphological analysis of the 6b oncogene-induced enation syndrome.Planta. 2016 Jan;243(1):131-48. doi: 10.1007/s00425-015-2387-0. Epub 2015 Sep 9. Planta. 2016. PMID: 26353911
References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

