Mycobacterium tuberculosis WhiB3 interacts with RpoV to affect host survival but is dispensable for in vivo growth
- PMID: 11880648
- PMCID: PMC122487
- DOI: 10.1073/pnas.052705399
Mycobacterium tuberculosis WhiB3 interacts with RpoV to affect host survival but is dispensable for in vivo growth
Abstract
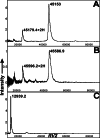
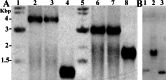
Previous work established that the principal sigma factor (RpoV) of virulent Mycobacterium bovis, a member of the Mycobacterium tuberculosis complex, restores virulence to an attenuated strain containing a point mutation (Arg-515-->His) in the 4.2 domain of RpoV. We used the 4.2 domain of RpoV as bait in a yeast two-hybrid screen of an M. tuberculosis H37Rv library and identified a putative transcription factor, WhiB3, which selectively interacts with the 4.2 domain of RpoV in virulent strains but not with the mutated (Arg-515-->His) allele. Infection of mice and guinea pigs with a M. tuberculosis H37Rv whiB3 deletion mutant strain showed that whiB3 is not necessary for in vivo bacterial replication in either animal model. In contrast, an M. bovis whiB3 deletion mutant was completely attenuated for growth in guinea pigs. However, we found that immunocompetent mice infected with the M. tuberculosis H37Rv whiB3 mutant strain had significantly longer mean survival times as compared with mice challenged with wild-type M. tuberculosis. Remarkably, the bacterial organ burdens of both mutant and wild-type infected mice were identical during the acute and persistent phases of infection. Our results imply that M. tuberculosis replication per se is not a sufficient condition for virulence in vivo. They also indicate a different role for M. bovis and M. tuberculosis whiB3 genes in pathogenesis generated in different animal models. We propose that M. tuberculosis WhiB3 functions as a transcription factor regulating genes that influence the immune response of the host.
Figures



Similar articles
-
Mutation of the principal sigma factor causes loss of virulence in a strain of the Mycobacterium tuberculosis complex.Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):8036-40. doi: 10.1073/pnas.92.17.8036. Proc Natl Acad Sci U S A. 1995. PMID: 7644534 Free PMC article.
-
Different susceptibility of two animal species infected with isogenic mutants of Mycobacterium bovis identifies phoT as having roles in tuberculosis virulence and phosphate transport.Microbiology (Reading). 2003 Nov;149(Pt 11):3203-3212. doi: 10.1099/mic.0.26469-0. Microbiology (Reading). 2003. PMID: 14600232
-
Examination of Mycobacterium tuberculosis sigma factor mutants using low-dose aerosol infection of guinea pigs suggests a role for SigC in pathogenesis.Microbiology (Reading). 2006 Jun;152(Pt 6):1591-1600. doi: 10.1099/mic.0.28591-0. Microbiology (Reading). 2006. PMID: 16735723
-
Extra and intracellular expression of Mycobacterium tuberculosis genes.Tuber Lung Dis. 1998;79(2):91-7. doi: 10.1054/tuld.1998.0010. Tuber Lung Dis. 1998. PMID: 10645446 Review.
-
Sigma factors of Mycobacterium tuberculosis.Tuber Lung Dis. 1997;78(3-4):175-83. doi: 10.1016/s0962-8479(97)90024-1. Tuber Lung Dis. 1997. PMID: 9713650 Review. No abstract available.
Cited by
-
Ancestral antibiotic resistance in Mycobacterium tuberculosis.Proc Natl Acad Sci U S A. 2005 Aug 23;102(34):12200-5. doi: 10.1073/pnas.0505446102. Epub 2005 Aug 15. Proc Natl Acad Sci U S A. 2005. PMID: 16103351 Free PMC article.
-
Differential gene expression in response to exposure to antimycobacterial agents and other stress conditions among seven Mycobacterium tuberculosis whiB-like genes.Antimicrob Agents Chemother. 2006 Aug;50(8):2836-41. doi: 10.1128/AAC.00295-06. Antimicrob Agents Chemother. 2006. PMID: 16870781 Free PMC article.
-
Mycobacterium tuberculosis MycP1 protease plays a dual role in regulation of ESX-1 secretion and virulence.Cell Host Microbe. 2010 Mar 18;7(3):210-20. doi: 10.1016/j.chom.2010.02.006. Cell Host Microbe. 2010. PMID: 20227664 Free PMC article.
-
WblAch, a pivotal activator of natamycin biosynthesis and morphological differentiation in Streptomyces chattanoogensis L10, is positively regulated by AdpAch.Appl Environ Microbiol. 2014 Nov;80(22):6879-87. doi: 10.1128/AEM.01849-14. Epub 2014 Aug 29. Appl Environ Microbiol. 2014. PMID: 25172865 Free PMC article.
-
Latent Tuberculosis: Models, Computational Efforts and the Pathogen's Regulatory Mechanisms during Dormancy.Front Bioeng Biotechnol. 2013 Aug 27;1:4. doi: 10.3389/fbioe.2013.00004. eCollection 2013. Front Bioeng Biotechnol. 2013. PMID: 25023946 Free PMC article. Review.
References
-
- Dye C, Scheele S, Dolin P, Pathania V, Raviglione M C. J Am Med Assoc. 1999;282:677–686. - PubMed
-
- Fine P E. Lancet. 1995;346:1339–1345. - PubMed
-
- Steyn A J C, Chan J, Mehra V. Curr Opin Infect Dis. 1999;12:415–424. - PubMed
-
- Glickman M S, Jacobs W R., Jr Cell. 2001;104:477–485. - PubMed
-
- Berthet F X, Lagranderie M, Gounon P, Laurent-Winter C, Ensergueix D, Chavarot P, Thouron F, Maranghi E, Pelicic V, Portnoi D, et al. Science. 1998;282:759–762. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

