Regulation of transferrin-mediated iron uptake by HFE, the protein defective in hereditary hemochromatosis
- PMID: 11867720
- PMCID: PMC122482
- DOI: 10.1073/pnas.042701499
Regulation of transferrin-mediated iron uptake by HFE, the protein defective in hereditary hemochromatosis
Abstract
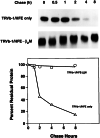
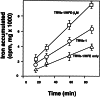
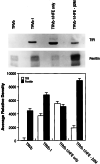
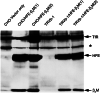
The protein defective in hereditary hemochromatosis, called HFE, is similar to MHC class I-type proteins and associates with beta2-microglobulin (beta2M). Its association with beta2M was previously shown to be necessary for its stability, normal intracellular processing, and cell surface expression in transfected COS cells. Here we use stably transfected Chinese hamster ovary cell lines expressing both HFE and beta2M or HFE alone to study the effects of beta2M on the stability and maturation of the HFE protein and on the role of HFE in transferrin receptor 1 (TfR1)-mediated iron uptake. In agreement with prior studies on other cell lines, we found that overexpression of HFE, without overexpressing beta2M, resulted in a decrease in TfR1dependent iron uptake and in lower iron levels in the cells, as evidenced by ferritin and TfR1 levels measured at steady state. However, overexpression of both HFE and beta2M had the reverse effect and resulted in an increase in TfR1-dependent iron uptake and increased iron levels in the cells. The HFE-beta2M complex did not affect the affinity of TfR1 for transferrin or the internalization rate of transferrin-bound TfR1. Instead, HFE-beta2M enhanced the rate of recycling of TfR1 and resulted in an increase in the steady-state level of TfR1 at the cell surface of stably transfected cells. We propose that Chinese hamster ovary cells provide a model to explain the effect of the HFE-beta2M complex in duodenal crypt cells, where the HFE-beta2M complex appears to facilitate the uptake of transferrin-bound iron to sense the level of body iron stores. Impairment of this process in duodenal crypt cells leads them to be iron poor and to signal the differentiating enterocytes to take up iron excessively after they mature into villus cells in the duodenum of hereditary hemochromatosis patients.
Figures
Similar articles
-
Association of HFE protein with transferrin receptor in crypt enterocytes of human duodenum.Proc Natl Acad Sci U S A. 1999 Feb 16;96(4):1579-84. doi: 10.1073/pnas.96.4.1579. Proc Natl Acad Sci U S A. 1999. PMID: 9990067 Free PMC article.
-
The hereditary hemochromatosis protein, HFE, lowers intracellular iron levels independently of transferrin receptor 1 in TRVb cells.Blood. 2005 Mar 15;105(6):2564-70. doi: 10.1182/blood-2004-03-1204. Epub 2004 Nov 4. Blood. 2005. PMID: 15528318
-
Overexpression of hemochromatosis protein, HFE, alters transferrin recycling process in human hepatoma cells.Biochim Biophys Acta. 2000 Apr 17;1496(2-3):221-31. doi: 10.1016/s0167-4889(00)00016-1. Biochim Biophys Acta. 2000. PMID: 10771090
-
Mechanisms and regulation of intestinal iron absorption.Blood Cells Mol Dis. 2002 Nov-Dec;29(3):384-99. doi: 10.1006/bcmd.2002.0578. Blood Cells Mol Dis. 2002. PMID: 12547229 Review.
-
Pumping iron: the strange partnership of the hemochromatosis protein, a class I MHC homolog, with the transferrin receptor.Traffic. 2001 Mar;2(3):167-74. doi: 10.1034/j.1600-0854.2001.020303.x. Traffic. 2001. PMID: 11260522 Review.
Cited by
-
Differential effects of basolateral and apical iron supply on iron transport in Caco-2 cells.Genes Nutr. 2015 May;10(3):463. doi: 10.1007/s12263-015-0463-5. Epub 2015 Apr 22. Genes Nutr. 2015. PMID: 25896409 Free PMC article.
-
Elevated transferrin receptor impairs T cell metabolism and function in systemic lupus erythematosus.Sci Immunol. 2023 Jan 13;8(79):eabq0178. doi: 10.1126/sciimmunol.abq0178. Epub 2023 Jan 13. Sci Immunol. 2023. PMID: 36638190 Free PMC article.
-
Prion protein and metal interaction: physiological and pathological implications.Curr Issues Mol Biol. 2010;12(2):99-107. Epub 2009 Sep 18. Curr Issues Mol Biol. 2010. PMID: 19767653 Free PMC article. Review.
-
Redox control of prion and disease pathogenesis.Antioxid Redox Signal. 2010 Jun 1;12(11):1271-94. doi: 10.1089/ars.2009.2628. Antioxid Redox Signal. 2010. PMID: 19803746 Free PMC article. Review.
-
Hereditary haemochromatosis gene (HFE) H63D mutation shows an association with abnormal sperm motility.Mol Biol Rep. 2009 Sep;36(7):1709-14. doi: 10.1007/s11033-008-9372-7. Epub 2008 Oct 10. Mol Biol Rep. 2009. PMID: 18846434
References
-
- Cartwright G E, Edwards C Q, Kravitz K, Skolnick M, Amos D B, Johnson A, Buskjaer L. N Engl J Med. 1979;301:175–179. - PubMed
-
- Cox T M, Lord D K. Eur J Haematol. 1989;42:113–125. - PubMed
-
- Bacon B R, Tavill A S. In: Hepatology: A Textbook of Liver Disease. Zakim D, Boyer T D, editors. Philadelphia: Saunders; 1996. pp. 1439–1472.
-
- McLaren G D, Nathanson M H, Jacobs A, Trevett D, Thomson W. J Lab Clin Med. 1991;117:390–401. - PubMed
-
- Feder J N, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy D A, Basava A, Dormishian F, Domingo R, Jr, Ellis M C, Fullan A, et al. Nat Genet. 1996;13:399–408. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

