Heparan sulfate proteoglycans are ligands for receptor protein tyrosine phosphatase sigma
- PMID: 11865065
- PMCID: PMC135600
- DOI: 10.1128/MCB.22.6.1881-1892.2002
Heparan sulfate proteoglycans are ligands for receptor protein tyrosine phosphatase sigma
Abstract
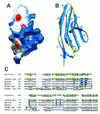
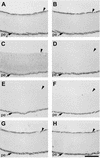
RPTPsigma is a cell adhesion molecule-like receptor protein tyrosine phosphatase involved in nervous system development. Its avian orthologue, known as cPTPsigma or CRYPalpha, promotes intraretinal axon growth and controls the morphology of growth cones. The molecular mechanisms underlying the functions of cPTPsigma are still to be determined, since neither its physiological ligand(s) nor its substrates have been described. Nevertheless, a major class of ligand(s) is present in the retinal basal lamina and glial endfeet, the potent native growth substrate for retinal axons. We demonstrate here that cPTPsigma is a heparin-binding protein and that its basal lamina ligands include the heparan sulfate proteoglycans (HSPGs) agrin and collagen XVIII. These molecules interact with high affinity with cPTPsigma in vitro, and this binding is totally dependent upon their heparan sulfate chains. Using molecular modelling and site-directed mutagenesis, a binding site for heparin and heparan sulfate was identified in the first immunoglobulin-like domain of cPTPsigma. HSPGs are therefore a novel class of heterotypic ligand for cPTPsigma, suggesting that cPTPsigma signaling in axons and growth cones is directly responsive to matrix-associated cues.
Figures
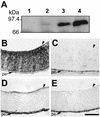

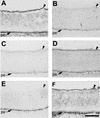
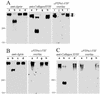
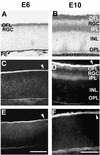


Similar articles
-
Isoform-specific binding of the tyrosine phosphatase PTPsigma to a ligand in developing muscle.Mol Cell Neurosci. 2003 Jan;22(1):37-48. doi: 10.1016/s1044-7431(02)00026-x. Mol Cell Neurosci. 2003. PMID: 12595237
-
Retinotectal ligands for the receptor tyrosine phosphatase CRYPalpha.Mol Cell Neurosci. 1999 Sep;14(3):225-40. doi: 10.1006/mcne.1999.0785. Mol Cell Neurosci. 1999. PMID: 10493824
-
Chick PTPsigma regulates the targeting of retinal axons within the optic tectum.J Neurosci. 2002 Jun 15;22(12):5024-33. doi: 10.1523/JNEUROSCI.22-12-05024.2002. J Neurosci. 2002. PMID: 12077198 Free PMC article.
-
The receptor tyrosine phosphatase CRYPalpha affects growth cone morphology.J Neurobiol. 2000 Aug;44(2):204-18. J Neurobiol. 2000. PMID: 10934323 Review.
-
Basement membrane proteoglycans: modulators Par Excellence of cancer growth and angiogenesis.Mol Cells. 2009 May 31;27(5):503-13. doi: 10.1007/s10059-009-0069-0. Epub 2009 May 15. Mol Cells. 2009. PMID: 19466598 Free PMC article. Review.
Cited by
-
A Synthetic Lethal Screen Identifies a Role for Lin-44/Wnt in C. elegans Embryogenesis.PLoS One. 2015 May 4;10(5):e0121397. doi: 10.1371/journal.pone.0121397. eCollection 2015. PLoS One. 2015. PMID: 25938228 Free PMC article.
-
The receptor protein tyrosine phosphatase LAR promotes R7 photoreceptor axon targeting by a phosphatase-independent signaling mechanism.Proc Natl Acad Sci U S A. 2009 Nov 17;106(46):19399-404. doi: 10.1073/pnas.0903961106. Epub 2009 Nov 4. Proc Natl Acad Sci U S A. 2009. PMID: 19889974 Free PMC article.
-
The Heparanase Regulatory Network in Health and Disease.Int J Mol Sci. 2021 Oct 14;22(20):11096. doi: 10.3390/ijms222011096. Int J Mol Sci. 2021. PMID: 34681753 Free PMC article. Review.
-
Functional regeneration beyond the glial scar.Exp Neurol. 2014 Mar;253:197-207. doi: 10.1016/j.expneurol.2013.12.024. Epub 2014 Jan 11. Exp Neurol. 2014. PMID: 24424280 Free PMC article. Review.
-
Postsynaptic TrkC and presynaptic PTPσ function as a bidirectional excitatory synaptic organizing complex.Neuron. 2011 Jan 27;69(2):287-303. doi: 10.1016/j.neuron.2010.12.024. Neuron. 2011. PMID: 21262467 Free PMC article.
References
-
- Baker, M. W., and E. R. Macagno. 2000. The role of a LAR-like receptor tyrosine phosphatase in growth cone collapse and mutual-avoidance by sibling processes. J. Neurobiol. 44:194-203. - PubMed
-
- Barford, D., A. K. Das, and M. P. Egloff. 1998. The structure and mechanism of protein phosphatases: insights into catalysis and regulation. Annu. Rev. Biophys. Biomol. Struct. 27:133-164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
