Modulation of the F-actin cytoskeleton by c-Abl tyrosine kinase in cell spreading and neurite extension
- PMID: 11864995
- PMCID: PMC2173320
- DOI: 10.1083/jcb.200110014
Modulation of the F-actin cytoskeleton by c-Abl tyrosine kinase in cell spreading and neurite extension
Abstract
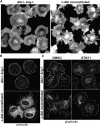
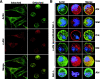
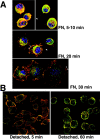
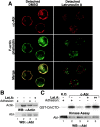
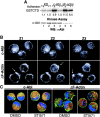
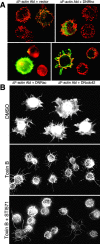
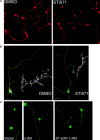
The nonreceptor tyrosine kinase encoded by the c-Abl gene has the unique feature of an F-actin binding domain (FABD). Purified c-Abl tyrosine kinase is inhibited by F-actin, and this inhibition can be relieved through mutation of its FABD. The c-Abl kinase is activated by physiological signals that also regulate the actin cytoskeleton. We show here that c-Abl stimulated the formation of actin microspikes in fibroblasts spreading on fibronectin. This function of c-Abl is dependent on kinase activity and is not shared by c-Src tyrosine kinase. The Abl-dependent F-actin microspikes occurred under conditions where the Rho-family GTPases were inhibited. The FABD-mutated c-Abl, which is active in detached fibroblasts, stimulated F-actin microspikes independent of cell attachment. Moreover, FABD-mutated c-Abl stimulated the formation of F-actin branches in neurites of rat embryonic cortical neurons. The reciprocal regulation between F-actin and the c-Abl tyrosine kinase may provide a self-limiting mechanism in the control of actin cytoskeleton dynamics.
Figures



Similar articles
-
Abl tyrosine kinase promotes dendrogenesis by inducing actin cytoskeletal rearrangements in cooperation with Rho family small GTPases in hippocampal neurons.J Neurosci. 2004 Sep 29;24(39):8510-21. doi: 10.1523/JNEUROSCI.1264-04.2004. J Neurosci. 2004. PMID: 15456825 Free PMC article.
-
Abl tyrosine kinase promotes dorsal ruffles but restrains lamellipodia extension during cell spreading on fibronectin.Mol Biol Cell. 2007 Oct;18(10):4143-54. doi: 10.1091/mbc.e07-01-0085. Epub 2007 Aug 8. Mol Biol Cell. 2007. PMID: 17686996 Free PMC article.
-
Regulation of F-actin-dependent processes by the Abl family of tyrosine kinases.J Cell Sci. 2003 Jul 1;116(Pt 13):2613-26. doi: 10.1242/jcs.00622. J Cell Sci. 2003. PMID: 12775773 Review.
-
Signaling function of PSGL-1 in neutrophil: tyrosine-phosphorylation-dependent and c-Abl-involved alteration in the F-actin-based cytoskeleton.J Cell Biochem. 2005 Feb 1;94(2):365-73. doi: 10.1002/jcb.20213. J Cell Biochem. 2005. PMID: 15526280
-
Breaking the neuronal sphere: regulation of the actin cytoskeleton in neuritogenesis.Nat Rev Neurosci. 2002 Sep;3(9):694-704. doi: 10.1038/nrn918. Nat Rev Neurosci. 2002. PMID: 12209118 Review.
Cited by
-
Non-Receptor Tyrosine Kinases: Their Structure and Mechanistic Role in Tumor Progression and Resistance.Cancers (Basel). 2024 Aug 2;16(15):2754. doi: 10.3390/cancers16152754. Cancers (Basel). 2024. PMID: 39123481 Free PMC article. Review.
-
Abl tyrosine kinase phosphorylates nonmuscle Myosin light chain kinase to regulate endothelial barrier function.Mol Biol Cell. 2010 Nov 15;21(22):4042-56. doi: 10.1091/mbc.E09-10-0876. Epub 2010 Sep 22. Mol Biol Cell. 2010. PMID: 20861316 Free PMC article.
-
c-Abl phosphorylates Dok1 to promote filopodia during cell spreading.J Cell Biol. 2004 May 24;165(4):493-503. doi: 10.1083/jcb.200312171. Epub 2004 May 17. J Cell Biol. 2004. PMID: 15148308 Free PMC article.
-
Abl activation regulates the dissociation of CAS from cytoskeletal vimentin by modulating CAS phosphorylation in smooth muscle.Am J Physiol Cell Physiol. 2010 Sep;299(3):C630-7. doi: 10.1152/ajpcell.00095.2010. Epub 2010 Jul 7. Am J Physiol Cell Physiol. 2010. PMID: 20610769 Free PMC article.
-
Emerging roles of the neural adaptor FE65 in neurite outgrowth.Neural Regen Res. 2018 Dec;13(12):2085-2086. doi: 10.4103/1673-5374.241449. Neural Regen Res. 2018. PMID: 30323128 Free PMC article. No abstract available.
References
-
- Bamburg, J. 1999. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu. Rev. Cell Dev. Biol. 15:185–230. - PubMed
-
- Bashaw, G., T. Kidd, D. Murray, T. Pawson, and C. Goodman. 2000. Repulsive axon guidance: Abelson and Enabled play opposing roles downstream of the roundabout receptor. Cell. 101:703–715. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

