Loss of the zymogen granule protein syncollin affects pancreatic protein synthesis and transport but not secretion
- PMID: 11839820
- PMCID: PMC134703
- DOI: 10.1128/MCB.22.5.1545-1554.2002
Loss of the zymogen granule protein syncollin affects pancreatic protein synthesis and transport but not secretion
Abstract
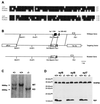
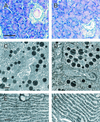
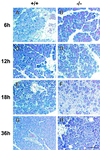
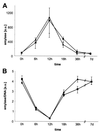
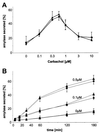
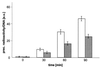
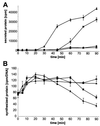
Syncollin is a small protein that is abundantly expressed in pancreatic acinar cells and that is tightly associated with the lumenal side of the zymogen granule membrane. To shed light on the hitherto unknown function of syncollin, we have generated syncollin-deficient mice. The mice are viable and show a normal pancreatic morphology as well as normal release kinetics in response to secretagogue stimulation. Although syncollin is highly enriched in zymogen granules, no change was found in the overall protein content and in the levels of chymotrypsin, trypsin, and amylase. However, syncollin-deficient mice reacted to caerulein hyperstimulation with a more severe pancreatitis. Furthermore, the rates of both protein synthesis and intracellular transport of secretory proteins were reduced. We conclude that syncollin plays a role in maturation and/or concentration of zymogens in zymogen granules.
Figures
Similar articles
-
Syncollin is required for efficient zymogen granule exocytosis.Biochem J. 2005 Feb 1;385(Pt 3):721-7. doi: 10.1042/BJ20041064. Biochem J. 2005. PMID: 15462671 Free PMC article.
-
Ectopic expression of syncollin in INS-1 beta-cells sorts it into granules and impairs regulated secretion.Biochemistry. 2005 Mar 22;44(11):4365-74. doi: 10.1021/bi048894d. Biochemistry. 2005. PMID: 15766266
-
Interaction of syncollin with GP-2, the major membrane protein of pancreatic zymogen granules, and association with lipid microdomains.Biochem J. 2002 Mar 1;362(Pt 2):433-42. doi: 10.1042/0264-6021:3620433. Biochem J. 2002. PMID: 11853552 Free PMC article.
-
Membrane targeting in secretion.Subcell Biochem. 2004;37:391-421. doi: 10.1007/978-1-4757-5806-1_12. Subcell Biochem. 2004. PMID: 15376628 Review.
-
The econobiology of pancreatic acinar cells granule inventory and the stealthy nano-machine behind it.Acta Histochem. 2016 Mar;118(2):194-202. doi: 10.1016/j.acthis.2015.11.011. Epub 2015 Dec 17. Acta Histochem. 2016. PMID: 26702787 Review.
Cited by
-
Biomarkers in the diagnosis of pancreatic cancer: Are we closer to finding the golden ticket?World J Gastroenterol. 2021 Jul 14;27(26):4045-4087. doi: 10.3748/wjg.v27.i26.4045. World J Gastroenterol. 2021. PMID: 34326612 Free PMC article. Review.
-
Integrated proteomic profiling of cell line conditioned media and pancreatic juice for the identification of pancreatic cancer biomarkers.Mol Cell Proteomics. 2011 Oct;10(10):M111.008599. doi: 10.1074/mcp.M111.008599. Epub 2011 Jun 7. Mol Cell Proteomics. 2011. PMID: 21653254 Free PMC article.
-
Vesicle-associated membrane protein (VAMP) cleavage by a new metalloprotease from the Brazilian scorpion Tityus serrulatus.J Biol Chem. 2010 Mar 5;285(10):7405-16. doi: 10.1074/jbc.M109.028365. Epub 2009 Dec 21. J Biol Chem. 2010. PMID: 20026600 Free PMC article.
-
Pancreatic gene expression during recovery after pancreatitis reveals unique transcriptome profiles.Sci Rep. 2018 Jan 23;8(1):1406. doi: 10.1038/s41598-018-19392-0. Sci Rep. 2018. PMID: 29362419 Free PMC article.
-
Global topology analysis of pancreatic zymogen granule membrane proteins.Mol Cell Proteomics. 2008 Dec;7(12):2323-36. doi: 10.1074/mcp.M700575-MCP200. Epub 2008 Aug 4. Mol Cell Proteomics. 2008. PMID: 18682380 Free PMC article.
References
-
- An, S. J., N. J. Hansen, A. Hodel, R. Jahn, and J. M. Edwardson. 2000. Analysis of the association of syncollin with the membrane of the pancreatic zymogen granule. J. Biol. Chem. 275:11306-11311. - PubMed
-
- Bergmeyer, H. U. 1974. Methoden der enzymatischen Analyse. Verlag Chemie, Weinheim/Bergstrasse, Germany.
-
- Bernfeld, P. 1955. Amylase, alpha and beta. Methods Enzymol. 1:31149-31158.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
