An endoplasmic reticulum protein, p180, is highly expressed in human cytomegalovirus-permissive cells and interacts with the tegument protein encoded by UL48
- PMID: 11836413
- PMCID: PMC153829
- DOI: 10.1128/jvi.76.5.2350-2362.2002
An endoplasmic reticulum protein, p180, is highly expressed in human cytomegalovirus-permissive cells and interacts with the tegument protein encoded by UL48
Abstract
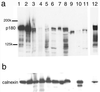
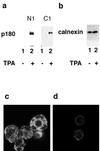
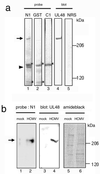
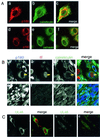
We have used a virus overlay assay to detect cellular proteins associated with human cytomegalovirus (HCMV) particles. The radiolabeled HCMV particles specifically bound to two host proteins with molecular sizes of 150 and 180 kDa. By a micro-amino-acid sequencing technique, the 180-kDa protein was identified as a human homologue of the ES130/p180 ribosome receptor (p180), which is an integral endoplasmic reticulum (ER) membrane protein possessing a very unique tandem repeat domain at its N-terminal region. The virus overlay assay using truncated p180 polypeptides revealed that HCMV binding to human p180 occurred through the N-terminal region. In HCMV-permissive cells the high level of expression of the human p180 protein was clearly observed regardless of cell type. Furthermore, we showed that p180 binds to the UL48 gene product, which is one of the predominant tegument proteins of HCMV and which is considered to be tightly associated with the capsid. The interaction between the two proteins was assumed to be specific and was observed both in vitro and in vivo. During the late phase of infection, the unique relocation of human p180 was observed, that is, to the juxtanuclear region, which appeared to be in the vicinity of the area where naked virions were frequently observed in an electron-microscopic study. Thus our data suggest that p180 interacts with the HCMV tegument, at least through pUL48, during the HCMV replication process. We discuss the possible role of the interaction between p180 and pUL48 in the intracellular transport of HCMV virions.
Figures




Similar articles
-
Tegument proteins of human cytomegalovirus.Microbiol Mol Biol Rev. 2008 Jun;72(2):249-65, table of contents. doi: 10.1128/MMBR.00040-07. Microbiol Mol Biol Rev. 2008. PMID: 18535146 Free PMC article. Review.
-
Human Cytomegalovirus UL48 Deubiquitinase Primarily Targets Innermost Tegument Proteins pp150 and Itself To Regulate Their Stability and Protects Virions from Inclusion of Ubiquitin Conjugates.J Virol. 2021 Nov 9;95(23):e0099121. doi: 10.1128/JVI.00991-21. Epub 2021 Sep 22. J Virol. 2021. PMID: 34549978 Free PMC article.
-
Involvement of the N-Terminal Deubiquitinating Protease Domain of Human Cytomegalovirus UL48 Tegument Protein in Autoubiquitination, Virion Stability, and Virus Entry.J Virol. 2016 Jan 13;90(6):3229-42. doi: 10.1128/JVI.02766-15. J Virol. 2016. PMID: 26764006 Free PMC article.
-
Identification of human cytomegalovirus genes important for biogenesis of the cytoplasmic virion assembly complex.J Virol. 2014 Aug;88(16):9086-99. doi: 10.1128/JVI.01141-14. Epub 2014 Jun 4. J Virol. 2014. PMID: 24899189 Free PMC article.
-
Role of human cytomegalovirus tegument proteins in virion assembly.Viruses. 2014 Feb 6;6(2):582-605. doi: 10.3390/v6020582. Viruses. 2014. PMID: 24509811 Free PMC article. Review.
Cited by
-
Targeting of herpesvirus capsid transport in axons is coupled to association with specific sets of tegument proteins.Proc Natl Acad Sci U S A. 2005 Apr 19;102(16):5832-7. doi: 10.1073/pnas.0500803102. Epub 2005 Mar 28. Proc Natl Acad Sci U S A. 2005. PMID: 15795370 Free PMC article.
-
p180 is involved in the interaction between the endoplasmic reticulum and microtubules through a novel microtubule-binding and bundling domain.Mol Biol Cell. 2007 Oct;18(10):3741-51. doi: 10.1091/mbc.e06-12-1125. Epub 2007 Jul 18. Mol Biol Cell. 2007. PMID: 17634287 Free PMC article.
-
Tegument proteins of human cytomegalovirus.Microbiol Mol Biol Rev. 2008 Jun;72(2):249-65, table of contents. doi: 10.1128/MMBR.00040-07. Microbiol Mol Biol Rev. 2008. PMID: 18535146 Free PMC article. Review.
-
Identification of functional domains within the essential large tegument protein pUL36 of pseudorabies virus.J Virol. 2007 Dec;81(24):13403-11. doi: 10.1128/JVI.01643-07. Epub 2007 Oct 10. J Virol. 2007. PMID: 17928337 Free PMC article.
-
Microtubule network facilitates nuclear targeting of human cytomegalovirus capsid.J Virol. 2003 Aug;77(15):8541-7. doi: 10.1128/jvi.77.15.8541-8547.2003. J Virol. 2003. PMID: 12857923 Free PMC article.
References
-
- Ando, Y., T. Iwasaki, T. Sata, S. Soushi, T. Kurata, and Y. Arao. 1997. Enhanced cytopathic effect of human cytomegalovirus on a retinal pigment epithelium cell line, K-1034, by serum-free medium. Arch. Virol. 142:1645-1658. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

