The zinc-finger proto-oncogene Gfi-1b is essential for development of the erythroid and megakaryocytic lineages
- PMID: 11825872
- PMCID: PMC155332
- DOI: 10.1101/gad.959102
The zinc-finger proto-oncogene Gfi-1b is essential for development of the erythroid and megakaryocytic lineages
Abstract
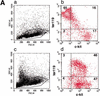
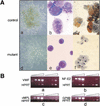
Gfi-1 and Gfi-1b are novel proto-oncogenes identified by retroviral insertional mutagenesis. By gene targeting, we establish that Gfi-1b is required for the development of two related blood lineages, erythroid and megakaryocytic, in mice. Gfi-1b(-/-) embryonic stem cells fail to contribute to red cells of adult chimeras. Gfi-1b(-/-) embryos exhibit delayed maturation of primitive erythrocytes and subsequently die with failure to produce definitive enucleated erythrocytes. The fetal liver of mutant mice contains erythroid and megakaryocytic precursors arrested in their development. Myelopoiesis is normal. Therefore, Gfi-1b is an essential transcriptional regulator of erythroid and megakaryocyte development.
Figures




Similar articles
-
Erythroid expansion mediated by the Gfi-1B zinc finger protein: role in normal hematopoiesis.Blood. 2002 Oct 15;100(8):2769-77. doi: 10.1182/blood-2002-01-0182. Blood. 2002. PMID: 12351384
-
Distinct, strict requirements for Gfi-1b in adult bone marrow red cell and platelet generation.J Exp Med. 2014 May 5;211(5):909-27. doi: 10.1084/jem.20131065. Epub 2014 Apr 7. J Exp Med. 2014. PMID: 24711581 Free PMC article.
-
Gfi-1B controls human erythroid and megakaryocytic differentiation by regulating TGF-beta signaling at the bipotent erythro-megakaryocytic progenitor stage.Blood. 2010 Apr 8;115(14):2784-95. doi: 10.1182/blood-2009-09-241752. Epub 2010 Feb 2. Blood. 2010. PMID: 20124515
-
Gfi-1 oncoproteins in hematopoiesis.Hematology. 2003 Oct;8(5):339-44. doi: 10.1080/10245330310001612116. Hematology. 2003. PMID: 14530176 Review.
-
Ontogeny of erythropoiesis in the mammalian embryo.Curr Top Dev Biol. 2008;82:1-22. doi: 10.1016/S0070-2153(07)00001-4. Curr Top Dev Biol. 2008. PMID: 18282515 Review.
Cited by
-
Inherited platelet dysfunction and hematopoietic transcription factor mutations.Platelets. 2017 Jan;28(1):20-26. doi: 10.1080/09537104.2016.1203400. Epub 2016 Jul 27. Platelets. 2017. PMID: 27463948 Free PMC article. Review.
-
GATA-1 forms distinct activating and repressive complexes in erythroid cells.EMBO J. 2005 Jul 6;24(13):2354-66. doi: 10.1038/sj.emboj.7600702. Epub 2005 May 26. EMBO J. 2005. PMID: 15920471 Free PMC article.
-
Massively parallel sequencing identifies the gene Megf8 with ENU-induced mutation causing heterotaxy.Proc Natl Acad Sci U S A. 2009 Mar 3;106(9):3219-24. doi: 10.1073/pnas.0813400106. Epub 2009 Feb 13. Proc Natl Acad Sci U S A. 2009. PMID: 19218456 Free PMC article.
-
Selective dissociation between LSD1 and GFI1B by a LSD1 inhibitor NCD38 induces the activation of ERG super-enhancer in erythroleukemia cells.Oncotarget. 2018 Apr 20;9(30):21007-21021. doi: 10.18632/oncotarget.24774. eCollection 2018 Apr 20. Oncotarget. 2018. PMID: 29765516 Free PMC article.
-
Transcriptional repressors, corepressors and chromatin modifying enzymes in T cell development.Cytokine. 2011 Mar;53(3):271-81. doi: 10.1016/j.cyto.2010.11.013. Epub 2010 Dec 16. Cytokine. 2011. PMID: 21163671 Free PMC article. Review.
References
-
- Fuchs B, Wagner T, Rossel N, Antoine M, Beug H, Niessing J. Structure and erythroid cell-restricted expression of a chicken cDNA encoding a novel zinc finger protein of the Cys + His class. Gene. 1997;195:277–284. - PubMed
-
- Gao Y, Sun Y, Frank KM, Dikkes P, Fujiwara Y, Seidl KJ, Sekiguchi JM, Rathbun GA, Swat W, Wang J, et al. A critical role for DNA end-joining proteins in both lymphogenesis and neurogenesis. Cell. 1998;95:891–902. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
