Novel role for CFTR in fluid absorption from the distal airspaces of the lung
- PMID: 11815669
- PMCID: PMC2233804
- DOI: 10.1085/jgp.119.2.199
Novel role for CFTR in fluid absorption from the distal airspaces of the lung
Abstract
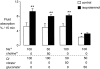
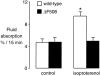
The active absorption of fluid from the airspaces of the lung is important for the resolution of clinical pulmonary edema. Although ENaC channels provide a major route for Na(+) absorption, the route of Cl(-) transport has been unclear. We applied a series of complementary approaches to define the role of Cl(-) transport in fluid clearance in the distal airspaces of the intact mouse lung, using wild-type and cystic fibrosis Delta F508 mice. Initial studies in wild-type mice showed marked inhibition of fluid clearance by Cl(-) channel inhibitors and Cl(-) ion substitution, providing evidence for a transcellular route for Cl(-) transport. In response to cAMP stimulation by isoproterenol, clearance was inhibited by the CFTR inhibitor glibenclamide in both wild-type mice and the normal human lung. Although isoproterenol markedly increased fluid absorption in wild-type mice, there was no effect in Delta F508 mice. Radioisotopic clearance studies done at 23 degrees C (to block active fluid absorption) showed approximately 20% clearance of (22)Na in 30 min both without and with isoproterenol. However, the clearance of (36)Cl was increased by 47% by isoproterenol in wild-type mice but was not changed in Delta F508 mice, providing independent evidence for involvement of CFTR in cAMP-stimulated Cl(-) transport. Further, CFTR played a major role in fluid clearance in a mouse model of acute volume-overload pulmonary edema. After infusion of saline (40% body weight), the lung wet-to-dry weight ratio increased by 28% in wild-type versus 64% in Delta F508 mice. These results provide direct evidence for a functionally important role for CFTR in the distal airspaces of the lung.
Figures
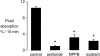
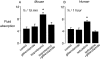

Similar articles
-
Chloride transport-driven alveolar fluid secretion is a major contributor to cardiogenic lung edema.Proc Natl Acad Sci U S A. 2013 Jun 18;110(25):E2308-16. doi: 10.1073/pnas.1216382110. Epub 2013 May 3. Proc Natl Acad Sci U S A. 2013. PMID: 23645634 Free PMC article.
-
Cystic fibrosis transmembrane conductance regulator (CFTR) and renal function.Wien Klin Wochenschr. 1997 Jun 27;109(12-13):457-64. Wien Klin Wochenschr. 1997. PMID: 9261986 Review.
-
Evidence for cystic fibrosis transmembrane conductance regulator-dependent sodium reabsorption in kidney, using Cftr(tm2cam) mice.J Physiol. 2000 Jul 1;526 Pt 1(Pt 1):27-34. doi: 10.1111/j.1469-7793.2000.00027.x. J Physiol. 2000. PMID: 10878096 Free PMC article.
-
Cftr and ENaC ion channels mediate NaCl absorption in the mouse submandibular gland.J Physiol. 2010 Feb 15;588(Pt 4):713-24. doi: 10.1113/jphysiol.2009.183541. Epub 2009 Dec 21. J Physiol. 2010. PMID: 20026617 Free PMC article.
-
Defects in processing and trafficking of cystic fibrosis transmembrane conductance regulator.Exp Nephrol. 2000 Nov-Dec;8(6):332-42. doi: 10.1159/000020687. Exp Nephrol. 2000. PMID: 11014930 Review.
Cited by
-
Association of cystic fibrosis transmembrane conductance regulator gene variants with acute lung injury in African American children with pneumonia*.Crit Care Med. 2012 Nov;40(11):3042-9. doi: 10.1097/CCM.0b013e31825d8f73. Crit Care Med. 2012. PMID: 22890249 Free PMC article.
-
RNA interference for CFTR attenuates lung fluid absorption at birth in rats.Respir Res. 2008 Jul 24;9(1):55. doi: 10.1186/1465-9921-9-55. Respir Res. 2008. PMID: 18652671 Free PMC article.
-
Bench-to-bedside review: the role of the alveolar epithelium in the resolution of pulmonary edema in acute lung injury.Crit Care. 2004 Dec;8(6):469-77. doi: 10.1186/cc2906. Epub 2004 Jun 30. Crit Care. 2004. PMID: 15566618 Free PMC article. Review.
-
CFTR fails to inhibit the epithelial sodium channel ENaC expressed in Xenopus laevis oocytes.J Physiol. 2005 May 1;564(Pt 3):671-82. doi: 10.1113/jphysiol.2004.079046. Epub 2005 Mar 3. J Physiol. 2005. PMID: 15746174 Free PMC article.
-
Neutrophils and their Fc gamma receptors are essential in a mouse model of transfusion-related acute lung injury.J Clin Invest. 2006 Jun;116(6):1615-23. doi: 10.1172/JCI27238. Epub 2006 May 18. J Clin Invest. 2006. PMID: 16710475 Free PMC article.
References
-
- Al-Bazzaz, F.J. 1994. Regulation of Na and Cl transport in sheep distal airways. Am. J. Physiol. 267:L193–L198. - PubMed
-
- Ballard, S.T., S.M. Schepens, J.C. Falcone, G.A. Meininger, and A.E. Taylor. 1992. Regional bioelectric properties of porcine airway epithelium. J. Appl. Physiol. 73:2021–2027. - PubMed
-
- Borok, Z., J. Liebler, R. Lubman, M. Foster, B. Zhou, X. Li, S. Zabski, and E.D. Crandall. 2002. Sodium transport proteins are expressed by rat alveolar epithlelial type I cells. Am. J. Physiol. Lung. In press. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

