Ultrastructural and biochemical alterations induced by 22,26-azasterol, a delta(24(25))-sterol methyltransferase inhibitor, on promastigote and amastigote forms of Leishmania amazonensis
- PMID: 11796362
- PMCID: PMC127026
- DOI: 10.1128/AAC.46.2.487-499.2002
Ultrastructural and biochemical alterations induced by 22,26-azasterol, a delta(24(25))-sterol methyltransferase inhibitor, on promastigote and amastigote forms of Leishmania amazonensis
Abstract
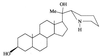
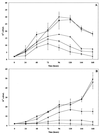
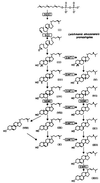
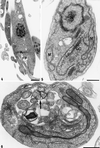
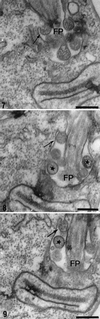
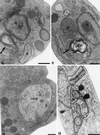
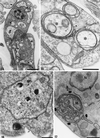
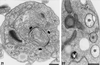
We report on the antiproliferative effects and the ultrastructural and biochemical alterations induced in vitro by 22,26-azasterol, a sterol Delta(24(25))-methyltransferase (24-SMT) inhibitor, on Leishmania amazonensis. When promastigotes and amastigotes were exposed to 100 nM 22,26-azasterol, complete growth arrest and cell lysis ensued after 72 (promastigotes) or 120 (amastigotes) h. Exposure of parasites to this azasterol led to the complete depletion of parasite endogenous sterols (episterol and 5-dehydroepisterol) and their replacement by 24-desalkyl sterols (zymosterol, cholesta-5,7,24-trien-3beta-ol, and cholesta-7,24-dien-3beta-ol), while 14-methyl-zymosterol and 4,14-dimethyl-zymosterol accumulated as a result of simultaneous incubation of the parasites with 22,26-azasterol and ketoconazole, a known inhibitor of the parasite's sterol C14-demethylase. These results confirmed that 24-SMT is the primary site of action of the azasterol. Profound changes were also observed in the phospholipid compositions of treated cells, in which a twofold reduction in the content of phosphatidylserine was observed; this was accompanied by a concomitant increase in the content of phosphatidylinositol. Transmission electron microscopy showed that 22,26-azasterol induced marked morphological changes, including mitochondrial swelling, invaginations of the inner mitochondrial membrane, and the appearance of large bodies containing concentric membranes. Other modifications included increases in the numbers of acidocalcisomes, megasomes, and lipid inclusions and the appearance of typical autophagic structures and cell body protrusions toward the flagellar pocket. We conclude that the dramatic alteration of the lipid composition of the parasite's membranes induced by the drug underlies the ultrastructural alterations that lead to the loss of cell viability and that 24-SMT inhibitors could be useful as selective antileishmanial agents.
Figures
Similar articles
-
Modification of the sterol composition of Trypanosoma (Schizotrypanum) cruzi epimastigotes by delta 24(25)-sterol methyl transferase inhibitors and their combinations with ketoconazole.Mol Biochem Parasitol. 1995 Jul;73(1-2):199-210. doi: 10.1016/0166-6851(95)00117-j. Mol Biochem Parasitol. 1995. PMID: 8577328
-
Sterol composition and biosynthesis in Trypanosoma cruzi amastigotes.Mol Biochem Parasitol. 1999 Oct 25;104(1):81-91. doi: 10.1016/s0166-6851(99)00129-2. Mol Biochem Parasitol. 1999. PMID: 10589983
-
Antiproliferative effects of delta 24(25) sterol methyl transferase inhibitors on Trypanosoma (Schizotrypanum) cruzi: in vitro and in vivo studies.Chemotherapy. 1996 Jul-Aug;42(4):294-307. doi: 10.1159/000239458. Chemotherapy. 1996. PMID: 8804798
-
Lipid biosynthesis pathways as chemotherapeutic targets in kinetoplastid parasites.Parasitology. 1997;114 Suppl:S91-9. Parasitology. 1997. PMID: 9309771 Review.
-
Morinda citrifolia Linn. fruit (Noni) juice induces an increase in NO production and death of Leishmania amazonensis amastigotes in peritoneal macrophages from BALB/c.Nitric Oxide. 2016 Aug 31;58:51-8. doi: 10.1016/j.niox.2016.06.004. Epub 2016 Jun 18. Nitric Oxide. 2016. PMID: 27328771 Review.
Cited by
-
Production and characterization of stable amphotericin-resistant amastigotes and promastigotes of Leishmania mexicana.Antimicrob Agents Chemother. 2005 Aug;49(8):3274-80. doi: 10.1128/AAC.49.8.3274-3280.2005. Antimicrob Agents Chemother. 2005. PMID: 16048936 Free PMC article.
-
Liposomal formulation of turmerone-rich hexane fractions from Curcuma longa enhances their antileishmanial activity.Biomed Res Int. 2014;2014:694934. doi: 10.1155/2014/694934. Epub 2014 Jun 18. Biomed Res Int. 2014. PMID: 25045693 Free PMC article.
-
Ultrastructural Changes and Death of Leishmania infantum Promastigotes Induced by Morinda citrifolia Linn. Fruit (Noni) Juice Treatment.Evid Based Complement Alternat Med. 2016;2016:5063540. doi: 10.1155/2016/5063540. Epub 2016 May 22. Evid Based Complement Alternat Med. 2016. PMID: 27313649 Free PMC article.
-
Antileishmanial activity of crude extract and coumarin from Calophyllum brasiliense leaves against Leishmania amazonensis.Parasitol Res. 2007 Aug;101(3):715-22. doi: 10.1007/s00436-007-0542-7. Epub 2007 May 5. Parasitol Res. 2007. PMID: 17483964
-
Benzylamines as highly potent inhibitors of the sterol biosynthesis pathway in Leishmania amazonensis leading to oxidative stress and ultrastructural alterations.Sci Rep. 2022 Jul 4;12(1):11313. doi: 10.1038/s41598-022-15449-3. Sci Rep. 2022. PMID: 35788652 Free PMC article.
References
-
- Ames, B., and D. Dubin. 1960. The role of polyamines in the neutralization of deoxyribonucleic acid. J. Biol. Chem. 235:769–775. - PubMed
-
- Barret-Bee, K., and N. S. Ryder. 1992. Biochemical aspects of ergosterol biosynthesis inhibition, p.410–436. In J. Sutcliffe and N. H. Georgopapadakou (ed.), Emerging targets in antibacterial and antifungal chemotherapy. Chapman & Hall, New York, N.Y.
-
- Beach, D. H., G. G. Holz, Jr., and G. E. Anekwe. 1979. Lipids of Leishmania promastigotes. J. Parasitol. 65:203–217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

