The glycosynapse
- PMID: 11773621
- PMCID: PMC117543
- DOI: 10.1073/pnas.012540899
The glycosynapse
Erratum in
- Proc Natl Acad Sci U S A 2002 Mar 5;99(5):3356
Abstract
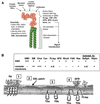
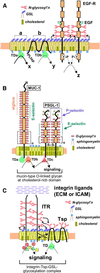
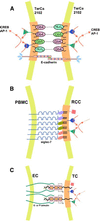
Physically distinguishable microdomains associated with various functional membrane proteins are one of the major current topics in cell biology. Glycosphingolipids present in such microdomains have been used as "markers;" however, the functional role of glycosyl epitopes in microdomains has received little attention. In this review, I have tried to summarize the evidence that glycosyl epitopes in microdomains mediate cell adhesion and signal transduction events that affect cellular phenotypes. Molecular assemblies that perform such functions are hereby termed "glycosynapse" in analogy to "immunological synapse," the membrane assembly of immunocyte adhesion and signaling. Three types of glycosynapses are so far distinguishable: (i) Glycosphingolipids organized with cytoplasmic signal transducers and proteolipid tetraspanin with or without growth factor receptors; (ii) transmembrane mucin-type glycoproteins with clustered O-linked glycoepitopes for cell adhesion and associated signal transducers at lipid domain; and (iii) N-glycosylated transmembrane adhesion receptors complexed with tetraspanin and gangliosides, as typically seen with the integrin-tetraspanin-ganglioside complex. The possibility is discussed that glycosynapses give rise to a high degree of diversity and complexity of phenotypes.
Figures
Similar articles
-
Functional role of glycosphingolipids and gangliosides in control of cell adhesion, motility, and growth, through glycosynaptic microdomains.Biochim Biophys Acta. 2008 Mar;1780(3):421-33. doi: 10.1016/j.bbagen.2007.10.008. Epub 2007 Oct 22. Biochim Biophys Acta. 2008. PMID: 17991443 Free PMC article. Review.
-
Glycosynapses: microdomains controlling carbohydrate-dependent cell adhesion and signaling.An Acad Bras Cienc. 2004 Sep;76(3):553-72. doi: 10.1590/s0001-37652004000300010. Epub 2004 Aug 23. An Acad Bras Cienc. 2004. PMID: 15334254 Review.
-
Shed gangliosides provide detergent-independent evidence for type-3 glycosynapses.Biochem Biophys Res Commun. 2007 Apr 27;356(1):306-11. doi: 10.1016/j.bbrc.2007.02.139. Epub 2007 Mar 5. Biochem Biophys Res Commun. 2007. PMID: 17350595
-
Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism.Cancer Res. 1996 Dec 1;56(23):5309-18. Cancer Res. 1996. PMID: 8968075 Review.
-
Glycosphingolipid-dependent cross-talk between glycosynapses interfacing tumor cells with their host cells: essential basis to define tumor malignancy.FEBS Lett. 2002 Oct 30;531(1):88-92. doi: 10.1016/s0014-5793(02)03479-8. FEBS Lett. 2002. PMID: 12401209 Review.
Cited by
-
Norovirus GII.4 virus-like particles recognize galactosylceramides in domains of planar supported lipid bilayers.Angew Chem Int Ed Engl. 2012 Nov 26;51(48):12020-4. doi: 10.1002/anie.201205972. Epub 2012 Oct 24. Angew Chem Int Ed Engl. 2012. PMID: 23097253 Free PMC article. No abstract available.
-
APOL1 polymorphism modulates sphingolipid profile of human podocytes.Glycoconj J. 2020 Dec;37(6):729-744. doi: 10.1007/s10719-020-09944-w. Epub 2020 Sep 11. Glycoconj J. 2020. PMID: 32915357 Free PMC article.
-
Direct quantitative determination of ceramide glycosylation in vivo: a new approach to evaluate cellular enzyme activity of glucosylceramide synthase.J Lipid Res. 2010 Apr;51(4):866-74. doi: 10.1194/jlr.D002949. Epub 2009 Oct 13. J Lipid Res. 2010. PMID: 19826105 Free PMC article.
-
Functional role of glycosphingolipids and gangliosides in control of cell adhesion, motility, and growth, through glycosynaptic microdomains.Biochim Biophys Acta. 2008 Mar;1780(3):421-33. doi: 10.1016/j.bbagen.2007.10.008. Epub 2007 Oct 22. Biochim Biophys Acta. 2008. PMID: 17991443 Free PMC article. Review.
-
Gangliosides GM1 and GM3 in the living cell membrane form clusters susceptible to cholesterol depletion and chilling.Mol Biol Cell. 2007 Jun;18(6):2112-22. doi: 10.1091/mbc.e07-01-0071. Epub 2007 Mar 28. Mol Biol Cell. 2007. PMID: 17392511 Free PMC article.
References
-
- Anderson R G W. Annu Rev Biochem. 1998;67:199–225. - PubMed
-
- Brown D A, London E. Annu Rev Cell Dev Biol. 1998;14:111–136. - PubMed
-
- Hakomori S, Handa K, Iwabuchi K, Yamamura S, Prinetti A. Glycobiology. 1998;8:xi–xviii. - PubMed
-
- Horejsí V, Drbal K, Cebecauer M, Cerný J, Brdicka T, Angelisová P, Stockinger H. Immunol Today. 1999;20:356–361. - PubMed
-
- Simons K, Ikonen E. Nature (London) 1997;387:569–572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

