Cytokines as adjuvants for the induction of anti-human immunodeficiency virus peptide immunoglobulin G (IgG) and IgA antibodies in serum and mucosal secretions after nasal immunization
- PMID: 11752142
- PMCID: PMC136814
- DOI: 10.1128/jvi.76.2.517-524.2002
Cytokines as adjuvants for the induction of anti-human immunodeficiency virus peptide immunoglobulin G (IgG) and IgA antibodies in serum and mucosal secretions after nasal immunization
Abstract
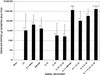
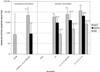
Safe and potent new adjuvants are needed for vaccines that are administered to mucosal surfaces. This study was performed to determine if interleukin-1alpha (IL-1alpha) combined with other proinflammatory cytokines provided mucosal adjuvant activity for induction of systemic and mucosal anti-human immunodeficiency virus (HIV) peptide antibody when intranasally administered with an HIV peptide immunogen. Nasal immunization of BALB/c mice with 10 microg of an HIV env peptide immunogen with IL-1alpha, IL-12, and IL-18 on days 0, 7, 14, and 28 induced peak serum anti-HIV peptide immunoglobulin G1 (IgG1) and IgA titers of 1:131,072 and 1:7,131, respectively (P = 0.05 versus no adjuvant). The use of cholera toxin (CT) as a mucosal adjuvant induced serum IgG1 and IgA titers of 1:32,768 and 1:776, respectively. The adjuvant combination of IL-1alpha, IL-12, and IL-18 induced anti-HIV peptide IgA titers of 1:1,176, 1:7,131, and 1:4,705 in saliva, fecal extracts and vaginal lavage, respectively. Titers induced by the use of CT as an adjuvant were 1:223, 1:1,176, and 1:675, respectively. These results indicate that the proinflammatory cytokines IL-1alpha, IL-12, and IL-18 can replace CT as a mucosal adjuvant for antibody induction and are important candidates for use as mucosal adjuvants with HIV and other vaccines.
Figures
Similar articles
-
Mucosal immunity to HIV-1: systemic and vaginal antibody responses after intranasal immunization with the HIV-1 C4/V3 peptide T1SP10 MN(A).J Immunol. 1996 Jul 1;157(1):462-72. J Immunol. 1996. PMID: 8683152
-
A comparative evaluation of nasal and parenteral vaccine adjuvants to elicit systemic and mucosal HIV-1 peptide-specific humoral immune responses in cynomolgus macaques.Vaccine. 2004 Sep 9;22(27-28):3774-88. doi: 10.1016/j.vaccine.2004.03.011. Vaccine. 2004. PMID: 15315859
-
Intranasal vaccination using interleukin-12 and cholera toxin subunit B as adjuvants to enhance mucosal and systemic immunity to human immunodeficiency virus type 1 glycoproteins.J Virol. 2003 May;77(10):5589-97. doi: 10.1128/jvi.77.10.5589-5597.2003. J Virol. 2003. PMID: 12719551 Free PMC article.
-
Induction of mucosal and systemic antibody and T-cell responses following prime-boost immunization with novel adjuvanted human immunodeficiency virus-1-vaccine formulations.J Gen Virol. 2011 Jan;92(Pt 1):128-40. doi: 10.1099/vir.0.023242-0. J Gen Virol. 2011. PMID: 21169215 Free PMC article.
-
Intranasal immunization with a plant virus expressing a peptide from HIV-1 gp41 stimulates better mucosal and systemic HIV-1-specific IgA and IgG than oral immunization.J Immunol Methods. 1998 Nov 1;220(1-2):93-103. doi: 10.1016/s0022-1759(98)00145-8. J Immunol Methods. 1998. PMID: 9839930
Cited by
-
Synthetic mast-cell granules as adjuvants to promote and polarize immunity in lymph nodes.Nat Mater. 2012 Jan 22;11(3):250-7. doi: 10.1038/nmat3222. Nat Mater. 2012. PMID: 22266469 Free PMC article.
-
In vitro and in vivo characterization of anthrax anti-protective antigen and anti-lethal factor monoclonal antibodies after passive transfer in a mouse lethal toxin challenge model to define correlates of immunity.Infect Immun. 2007 Nov;75(11):5443-52. doi: 10.1128/IAI.00529-07. Epub 2007 Aug 20. Infect Immun. 2007. PMID: 17709410 Free PMC article.
-
A comparison of non-toxin vaccine adjuvants for their ability to enhance the immunogenicity of nasally-administered anthrax recombinant protective antigen.Vaccine. 2013 Mar 1;31(11):1480-9. doi: 10.1016/j.vaccine.2013.01.012. Epub 2013 Jan 23. Vaccine. 2013. PMID: 23352329 Free PMC article.
-
New players in cytokine control of HIV infection.Curr HIV/AIDS Rep. 2008 Feb;5(1):27-32. doi: 10.1007/s11904-008-0005-5. Curr HIV/AIDS Rep. 2008. PMID: 18417032 Review.
-
The mast cell activator compound 48/80 is safe and effective when used as an adjuvant for intradermal immunization with Bacillus anthracis protective antigen.Vaccine. 2009 Jun 2;27(27):3544-52. doi: 10.1016/j.vaccine.2009.03.069. Epub 2009 Apr 14. Vaccine. 2009. PMID: 19464533 Free PMC article.
References
-
- Ahlers, J. D., N. Dunlop, D. W. Alling, P. L. Nara, and J. A. Berzofsky. 1997. Cytokine-in-adjuvant steering of the immune response phenotype to Hiv-1 vaccine constructs — granulocyte-macrophage colony-stimulating factor and Tnf-alpha synergize with Il-12 to enhance induction of cytotoxic T lymphocytes. J. Immunol. 158:3947–3958. - PubMed
-
- Anonymous. 2000. AIDS epidemic update: December 2000. Joint United Nations Programme on HIV/AIDS (UNAIDS). UNAIDS, Geneva, Switzerland. - PubMed
-
- Anonymous. 2000. Report on the global HIV/AIDS epidemic. Joint United Nations Programme on HIV/AIDS, Geneva, Switzerland.
-
- Apter, F. M., W. I. Lencer, R. A. Finkelstein, J. J. Mekalanos, and M. R. Neutra. 1993. Monoclonal immunoglobulin A antibodies directed against cholera toxin prevent the toxin-induced chloride secretory response and block toxin binding to intestinal epithelial cells in vitro. Infect. Immun. 61:5271–5278. - PMC - PubMed
-
- Atkins, M. B., M. J. Robertson, M. Gordon, M. T. Lotze, M. DeCoste, J. S. DuBois, J. Ritz, A. B. Sandler, H. D. Edington, P. D. Garzone, J. W. Mier, C. M. Canning, L. Battiato, H. Tahara, and M. L. Sherman. 1997. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin. Cancer Res. 3:409–417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

