Respiration-dependent utilization of sugars in yeasts: a determinant role for sugar transporters
- PMID: 11751819
- PMCID: PMC139568
- DOI: 10.1128/JB.184.2.427-432.2002
Respiration-dependent utilization of sugars in yeasts: a determinant role for sugar transporters
Abstract
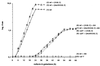
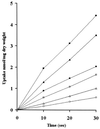
In many yeast species, including Kluyveromyces lactis, growth on certain sugars (such as galactose, raffinose, and maltose) occurs only under respiratory conditions. If respiration is blocked by inhibitors, mutation, or anaerobiosis, growth does not take place. This apparent dependence on respiration for the utilization of certain sugars has often been suspected to be associated with the mechanism of the sugar uptake step. We hypothesized that in many yeast species, the permease activities for these sugars are not sufficient to ensure the high substrate flow that is necessary for fermentative growth. By introducing additional sugar permease genes, we have obtained K. lactis strains that were capable of growing on galactose and raffinose in the absence of respiration. High dosages of both the permease and maltase genes were indeed necessary for K. lactis cells to grow on maltose in the absence of respiration. These results strongly suggest that the sugar uptake step is the major bottleneck in the fermentative assimilation of certain sugars in K. lactis and probably in many other yeasts.
Figures

Similar articles
-
Sugar transport in Saccharomyces cerevisiae.FEMS Microbiol Rev. 1993 Apr;10(3-4):229-42. doi: 10.1016/0378-1097(93)90598-v. FEMS Microbiol Rev. 1993. PMID: 8318258 Review.
-
A PEST-like sequence in the N-terminal cytoplasmic domain of Saccharomyces maltose permease is required for glucose-induced proteolysis and rapid inactivation of transport activity.Biochemistry. 2000 Apr 18;39(15):4518-26. doi: 10.1021/bi992455a. Biochemistry. 2000. PMID: 10758001
-
The Kluyver effect revisited.FEMS Yeast Res. 2003 Jun;3(4):327-31. doi: 10.1016/S1567-1356(03)00112-0. FEMS Yeast Res. 2003. PMID: 12748045 Review.
-
IMP2, a nuclear gene controlling the mitochondrial dependence of galactose, maltose and raffinose utilization in Saccharomyces cerevisiae.Yeast. 1992 Feb;8(2):83-93. doi: 10.1002/yea.320080203. Yeast. 1992. PMID: 1561839
-
Galactose transport in Kluyveromyces lactis: major role of the glucose permease Hgt1.FEMS Yeast Res. 2006 Dec;6(8):1235-42. doi: 10.1111/j.1567-1364.2006.00107.x. FEMS Yeast Res. 2006. PMID: 17156020
Cited by
-
Role of hexose transport in control of glycolytic flux in Saccharomyces cerevisiae.Appl Environ Microbiol. 2004 Sep;70(9):5323-30. doi: 10.1128/AEM.70.9.5323-5330.2004. Appl Environ Microbiol. 2004. PMID: 15345416 Free PMC article.
-
Evolution and functional diversification of yeast sugar transporters.Essays Biochem. 2023 Sep 13;67(5):811-827. doi: 10.1042/EBC20220233. Essays Biochem. 2023. PMID: 36928992 Free PMC article.
-
Opuntia ficus-indica cladodes as feedstock for ethanol production by Kluyveromyces marxianus and Saccharomyces cerevisiae.World J Microbiol Biotechnol. 2014 Dec;30(12):3173-83. doi: 10.1007/s11274-014-1745-6. Epub 2014 Sep 24. World J Microbiol Biotechnol. 2014. PMID: 25248867 Free PMC article.
-
Enhancing the Heterologous Fructosyltransferase Activity of Kluyveromyces lactis: Developing a Scaled-Up Process and Abolishing Invertase by CRISPR/Cas9 Genome Editing.Front Bioeng Biotechnol. 2020 Nov 25;8:607507. doi: 10.3389/fbioe.2020.607507. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 33324627 Free PMC article.
-
An example of the prisoner's dilemma in biochemistry.Naturwissenschaften. 2003 Jul;90(7):327-31. doi: 10.1007/s00114-003-0434-3. Epub 2003 Jun 26. Naturwissenschaften. 2003. PMID: 12883777
References
-
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410. - PubMed
-
- Barnett, J. A. 1976. The utilization of sugars by yeasts. Adv. Carbohydr. Chem. Biochem. 32:125–234. - PubMed
-
- Barnett, J. A. 1992. Some controls on oligosaccharide utilization by yeasts: the physiological basis of the Kluyver effect. FEMS Microbiol. Lett. 79:371–378. - PubMed
-
- Bianchi, M. M., C. Falcone, X. J. Chen, M. Wésolowski-Louvel, L. Frontali, and H. Fukuhara. 1987. Transformation of the yeast Kluyveromyces lactis by new vectors derived from the 1.6 μm circular plasmid pkD1. Curr. Genet. 12:185–192.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

