Role for the related poly(ADP-Ribose) polymerases tankyrase 1 and 2 at human telomeres
- PMID: 11739745
- PMCID: PMC134233
- DOI: 10.1128/MCB.22.1.332-342.2002
Role for the related poly(ADP-Ribose) polymerases tankyrase 1 and 2 at human telomeres
Abstract
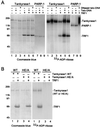
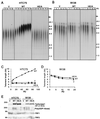
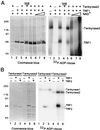
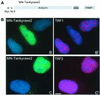
Telomere maintenance is essential for the continuous growth of tumor cells. In most human tumors telomeres are maintained by telomerase, a specialized reverse transcriptase. Tankyrase 1, a human telomeric poly(ADP-ribose) polymerase (PARP), positively regulates telomere length through its interaction with TRF1, a telomeric DNA-binding protein. Tankyrase 1 ADP-ribosylates TRF1, inhibiting its binding to telomeric DNA. Overexpression of tankyrase 1 in the nucleus promotes telomere elongation, suggesting that tankyrase 1 regulates access of telomerase to the telomeric complex. The recent identification of a closely related homolog of tankyrase 1, tankyrase 2, opens the possibility for a second PARP at telomeres. We therefore sought to establish the role of tankyrase 1 at telomeres and to determine if tankyrase 2 might have a telomeric function. We show that endogenous tankyrase 1 is a component of the human telomeric complex. We demonstrate that telomere elongation by tankyrase 1 requires the catalytic activity of the PARP domain and does not occur in telomerase-negative primary human cells. To investigate a potential role for tankyrase 2 at telomeres, recombinant tankyrase 2 was subjected to an in vitro PARP assay. Tankyrase 2 poly(ADP-ribosyl)ated itself and TRF1. Overexpression of tankyrase 2 in the nucleus released endogenous TRF1 from telomeres. These findings establish tankyrase 2 as a bona fide PARP, with itself and TRF1 as acceptors of ADP-ribosylation, and suggest the possibility of a role for tankyrase 2 at telomeres.
Figures




Similar articles
-
Tankyrase promotes telomere elongation in human cells.Curr Biol. 2000 Oct 19;10(20):1299-302. doi: 10.1016/s0960-9822(00)00752-1. Curr Biol. 2000. PMID: 11069113
-
The telomeric poly(ADP-ribose) polymerase, tankyrase 1, contains multiple binding sites for telomeric repeat binding factor 1 (TRF1) and a novel acceptor, 182-kDa tankyrase-binding protein (TAB182).J Biol Chem. 2002 Apr 19;277(16):14116-26. doi: 10.1074/jbc.M112266200. Epub 2002 Feb 19. J Biol Chem. 2002. PMID: 11854288
-
Tankyrase, a poly(ADP-ribose) polymerase at human telomeres.Science. 1998 Nov 20;282(5393):1484-7. doi: 10.1126/science.282.5393.1484. Science. 1998. PMID: 9822378
-
The telomeric PARP, tankyrases, as targets for cancer therapy.Br J Cancer. 2006 Feb 13;94(3):341-5. doi: 10.1038/sj.bjc.6602951. Br J Cancer. 2006. PMID: 16421589 Free PMC article. Review.
-
A cellular survival switch: poly(ADP-ribosyl)ation stimulates DNA repair and silences transcription.Bioessays. 2001 Jun;23(6):543-8. doi: 10.1002/bies.1074. Bioessays. 2001. PMID: 11385634 Review.
Cited by
-
Tankyrase-1 overexpression reduces genotoxin-induced cell death by inhibiting PARP1.Mol Cell Biochem. 2005 Aug;276(1-2):183-92. doi: 10.1007/s11010-005-4059-z. Mol Cell Biochem. 2005. PMID: 16132700
-
Generation and characterization of telomere length maintenance in tankyrase 2-deficient mice.Mol Cell Biol. 2006 Mar;26(6):2037-43. doi: 10.1128/MCB.26.6.2037-2043.2006. Mol Cell Biol. 2006. PMID: 16507984 Free PMC article.
-
Protein requirements for sister telomere association in human cells.EMBO J. 2007 Nov 28;26(23):4867-78. doi: 10.1038/sj.emboj.7601903. Epub 2007 Oct 25. EMBO J. 2007. PMID: 17962804 Free PMC article.
-
Human telomerase and its regulation.Microbiol Mol Biol Rev. 2002 Sep;66(3):407-25, table of contents. doi: 10.1128/MMBR.66.3.407-425.2002. Microbiol Mol Biol Rev. 2002. PMID: 12208997 Free PMC article. Review.
-
Telomerase in Cancer: Function, Regulation, and Clinical Translation.Cancers (Basel). 2022 Feb 5;14(3):808. doi: 10.3390/cancers14030808. Cancers (Basel). 2022. PMID: 35159075 Free PMC article. Review.
References
-
- Ame, J., E. Jacobson, and M. Jacobson. 2001. ADP-ribose polymer metabolism, p. 1–34. In G. D. Murcia, and S. Shall (ed.), From DNA damage and stress signalling to cell death: poly(ADP-ribosyl)ation reactions. Oxford University Press, Oxford, England.
-
- Bilaud, T., C. Brun, K. Ancelin, C. E. Koering, T. Laroche, and E. Gilson. 1997. Telomeric localization of TRF2, a novel human telobox protein. Nat. Genet. 17:236–239. - PubMed
-
- Broccoli, D., A. Smogorzewska, L. Chong, and T. de Lange. 1997. Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2. Nat. Genet. 17:231–235. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
