Sialylated O-glycans and sulfated tyrosines in the NH2-terminal domain of CC chemokine receptor 5 contribute to high affinity binding of chemokines
- PMID: 11733580
- PMCID: PMC2193526
- DOI: 10.1084/jem.194.11.1661
Sialylated O-glycans and sulfated tyrosines in the NH2-terminal domain of CC chemokine receptor 5 contribute to high affinity binding of chemokines
Abstract
The chemokine receptor CCR5 plays an important role in leukocyte chemotaxis and activation, and also acts as a coreceptor for human and simian immunodeficiency viruses (HIV-1, HIV-2, and SIV). We provide evidence that CCR5 is O-glycosylated on serine 6 in the NH2 terminus. The O-linked glycans, particularly sialic acid moieties, significantly contribute to binding of the chemokine ligands. By contrast, removal of O-linked oligosaccharide exerted little effect on HIV-1 infection. Sulfation of specific tyrosine residues in the CCR5 NH2 terminus was important for efficient beta-chemokine binding. Thus, as has been observed for the binding of selectins and their ligands, O-linked carbohydrates and tyrosine sulfates play major roles in promoting the interaction of chemokines with CCR5. The resulting flexible arrays of negative charges on the CCR5 surface may allow specific, high-affinity interactions with diverse chemokine ligands. Although this is the first example of O-linked oligosaccharides and tyrosine sulfates playing a role in chemokine binding, the high density of serines, threonines and tyrosines in the N-termini of many CC chemokine receptors suggests that these posttranslational modifications may commonly contribute to chemokine binding.
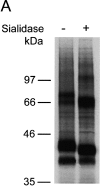
Figures











Similar articles
-
Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry.Cell. 1999 Mar 5;96(5):667-76. doi: 10.1016/s0092-8674(00)80577-2. Cell. 1999. PMID: 10089882
-
Allovalency observed by transferred NOE: interactions of sulfated tyrosine residues in the N-terminal segment of CCR5 with the CCL5 chemokine.FEBS J. 2021 Mar;288(5):1648-1663. doi: 10.1111/febs.15503. Epub 2020 Sep 8. FEBS J. 2021. PMID: 32814359
-
Molecular anatomy of CCR5 engagement by physiologic and viral chemokines and HIV-1 envelope glycoproteins: differences in primary structural requirements for RANTES, MIP-1 alpha, and vMIP-II Binding.J Mol Biol. 2001 Nov 9;313(5):1181-93. doi: 10.1006/jmbi.2001.5086. J Mol Biol. 2001. PMID: 11700073
-
Involvement of both the V2 and V3 regions of the CCR5-tropic human immunodeficiency virus type 1 envelope in reduced sensitivity to macrophage inflammatory protein 1alpha.J Virol. 2000 Feb;74(4):1787-93. doi: 10.1128/jvi.74.4.1787-1793.2000. J Virol. 2000. PMID: 10644351 Free PMC article.
-
The core domain of chemokines binds CCR5 extracellular domains while their amino terminus interacts with the transmembrane helix bundle.J Biol Chem. 2003 Feb 14;278(7):5179-87. doi: 10.1074/jbc.M205684200. Epub 2002 Dec 3. J Biol Chem. 2003. PMID: 12466283
Cited by
-
Cosmc controls B cell homing.Nat Commun. 2020 Aug 10;11(1):3990. doi: 10.1038/s41467-020-17765-6. Nat Commun. 2020. PMID: 32778659 Free PMC article.
-
Tyrosine sulfation and O-glycosylation of chemoattractant receptor GPR15 differentially regulate interaction with GPR15L.J Cell Sci. 2021 Apr 15;134(8):jcs247833. doi: 10.1242/jcs.247833. Epub 2021 Apr 22. J Cell Sci. 2021. PMID: 33758080 Free PMC article.
-
Transmitted/founder and chronic HIV-1 envelope proteins are distinguished by differential utilization of CCR5.J Virol. 2013 Mar;87(5):2401-11. doi: 10.1128/JVI.02964-12. Epub 2012 Dec 26. J Virol. 2013. PMID: 23269796 Free PMC article.
-
US28: HCMV's Swiss Army Knife.Viruses. 2018 Aug 20;10(8):445. doi: 10.3390/v10080445. Viruses. 2018. PMID: 30127279 Free PMC article. Review.
-
Regulation of C-C chemokine receptor 5 (CCR5) stability by Lys197 and by transmembrane protein aptamers that target it for lysosomal degradation.J Biol Chem. 2018 Jun 8;293(23):8787-8801. doi: 10.1074/jbc.RA117.001067. Epub 2018 Apr 20. J Biol Chem. 2018. PMID: 29678881 Free PMC article.
References
-
- Baggiolini, M. 1998. Chemokines and leukocyte traffic. Nature. 392:565–568. - PubMed
-
- Strieter, R.M., T.J. Standiford, G.B. Huffnagle, L.M. Colletti, N.W. Lukacs, and S.L. Kunkel. 1996. “The good, the bad, and the ugly.” The role of chemokines in models of human disease. J. Immunol. 156:3583–3586. - PubMed
-
- Rollins, B.J. 1997. Chemokines. Blood. 90:909–928. - PubMed
-
- Alkhatib, G., C. Combadiere, C.C. Broder, Y. Feng, P.E. Kennedy, P.M. Murphy, and E.A. Berger. 1996. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 272:1955–1958. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

