Apoptosis-associated release of Smac/DIABLO from mitochondria requires active caspases and is blocked by Bcl-2
- PMID: 11726499
- PMCID: PMC125329
- DOI: 10.1093/emboj/20.23.6627
Apoptosis-associated release of Smac/DIABLO from mitochondria requires active caspases and is blocked by Bcl-2
Abstract
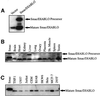
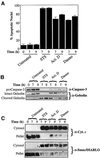
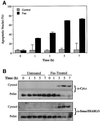
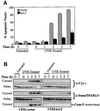
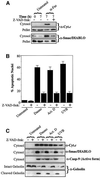
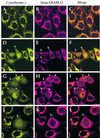
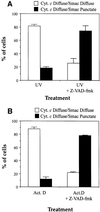
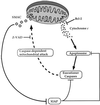
Smac/DIABLO is a mitochondrial protein that potentiates some forms of apoptosis, possibly by neutralizing one or more members of the IAP family of apoptosis inhibitory proteins. Smac has been shown to exit mitochondria and enter the cytosol during apoptosis triggered by UV- or gamma-irradiation. Here, we report that Smac/DIABLO export from mitochondria into the cytosol is provoked by cytotoxic drugs and DNA damage, as well as by ligation of the CD95 death receptor. Mitochondrial efflux of Smac/DIABLO, in response to a variety of pro-apoptotic agents, was profoundly inhibited in Bcl-2-overexpressing cells. Thus, in addition to modulating apoptosis-associated mitochondrial cytochrome c release, Bcl-2 also regulates Smac release, suggesting that both molecules may escape via the same route. However, whereas cell stress-associated mitochondrial cytochrome c release was largely caspase independent, release of Smac/DIABLO in response to the same stimuli was blocked by a broad-spectrum caspase inhibitor. This suggests that apoptosis-associated cytochrome c and Smac/DIABLO release from mitochondria do not occur via the same mechanism. Rather, Smac/DIABLO efflux from mitochondria is a caspase-catalysed event that occurs downstream of cytochrome c release.
Figures



Similar articles
-
Real-time single cell analysis of Smac/DIABLO release during apoptosis.J Cell Biol. 2003 Sep 15;162(6):1031-43. doi: 10.1083/jcb.200303123. J Cell Biol. 2003. PMID: 12975347 Free PMC article.
-
Cleavage of BID during cytotoxic drug and UV radiation-induced apoptosis occurs downstream of the point of Bcl-2 action and is catalysed by caspase-3: a potential feedback loop for amplification of apoptosis-associated mitochondrial cytochrome c release.Cell Death Differ. 2000 Jun;7(6):556-65. doi: 10.1038/sj.cdd.4400689. Cell Death Differ. 2000. PMID: 10822279
-
Involvement of proapoptotic molecules Bax and Bak in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced mitochondrial disruption and apoptosis: differential regulation of cytochrome c and Smac/DIABLO release.Cancer Res. 2003 Apr 1;63(7):1712-21. Cancer Res. 2003. PMID: 12670926
-
Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra.J Neuropathol Exp Neurol. 2003 Apr;62(4):329-39. doi: 10.1093/jnen/62.4.329. J Neuropathol Exp Neurol. 2003. PMID: 12722825 Review.
-
Smac/DIABLO and colon cancer.Anticancer Agents Med Chem. 2007 Jul;7(4):467-73. doi: 10.2174/187152007781058631. Anticancer Agents Med Chem. 2007. PMID: 17630921 Review.
Cited by
-
Curcumin suppressed anti-apoptotic signals and activated cysteine proteases for apoptosis in human malignant glioblastoma U87MG cells.Neurochem Res. 2007 Dec;32(12):2103-13. doi: 10.1007/s11064-007-9376-z. Epub 2007 Jun 12. Neurochem Res. 2007. PMID: 17562168
-
Insights into the mitochondrial signaling pathway: what lessons for chemotherapy?J Clin Immunol. 2003 Mar;23(2):73-80. doi: 10.1023/a:1022541009662. J Clin Immunol. 2003. PMID: 12757259 Review.
-
Mono- or double-site phosphorylation distinctly regulates the proapoptotic function of Bax.PLoS One. 2010 Oct 14;5(10):e13393. doi: 10.1371/journal.pone.0013393. PLoS One. 2010. PMID: 20976235 Free PMC article.
-
Metalloproteinase dependent reduction of cell surface cluster determinants upon the induction of apoptosis.Int J Oncol. 2014 May;44(5):1539-50. doi: 10.3892/ijo.2014.2344. Epub 2014 Mar 13. Int J Oncol. 2014. PMID: 24626736 Free PMC article.
-
SMAC-armed vaccinia virus induces both apoptosis and necroptosis and synergizes the efficiency of vinblastine in HCC.Hum Cell. 2014 Oct;27(4):162-71. doi: 10.1007/s13577-014-0093-z. Epub 2014 Apr 26. Hum Cell. 2014. PMID: 24771354
References
-
- Adrain C. and Martin,S.J. (2001) The mitochondrial apoptosome: a killer unleashed by the cytochrome seas. Trends Biochem. Sci., 26, 390–397. - PubMed
-
- Adrain C., Slee,E.A., Harte,M.T. and Martin,S.J. (1999) Regulation of apoptotic protease activating factor-1 oligomerization and apoptosis by the WD-40 repeat region. J. Biol. Chem., 274, 20855–20860. - PubMed
-
- Antonsson B., Montessuit,S., Sanchez,B. and Martinou,J.C. (2001) Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem., 276, 11615–11623. - PubMed
-
- Bossy-Wetzel E. and Green,D.R. (1999) Caspases induce cytochrome c release from mitochondria by activating cytosolic factors. J. Biol. Chem., 274, 17484–17490. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

