Assembly of an FtsZ mutant deficient in GTPase activity has implications for FtsZ assembly and the role of the Z ring in cell division
- PMID: 11717278
- PMCID: PMC95568
- DOI: 10.1128/JB.183.24.7190-7197.2001
Assembly of an FtsZ mutant deficient in GTPase activity has implications for FtsZ assembly and the role of the Z ring in cell division
Abstract
FtsZ, the ancestral homologue of eukaryotic tubulins, assembles into the Z ring, which is required for cytokinesis in prokaryotic cells. Both FtsZ and tubulin have a GTPase activity associated with polymerization. Interestingly, the ftsZ2 mutant is viable, although the FtsZ2 mutant protein has dramatically reduced GTPase activity due to a glycine-for-aspartic acid substitution within the synergy loop. In this study, we have examined the properties of FtsZ2 and found that the reduced GTPase activity is not enhanced by DEAE-dextran-induced assembly, indicating it has a defective catalytic site. In the absence of DEAE-dextran, FtsZ2 fails to assemble unless supplemented with wild-type FtsZ. FtsZ has to be at or above the critical concentration for copolymerization to occur, indicating that FtsZ is nucleating the copolymers. The copolymers formed are relatively stable and appear to be stabilized by a GTP-cap. These results indicate that FtsZ2 cannot nucleate assembly in vitro, although it must in vivo. Furthermore, the stability of FtsZ-FtsZ2 copolymers argues that FtsZ2 polymers would be stable, suggesting that stable FtsZ polymers are able to support cell division.
Figures
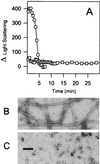
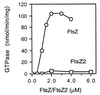
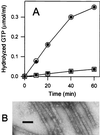
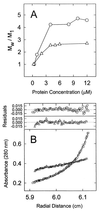


Similar articles
-
Dynamic assembly of FtsZ regulated by GTP hydrolysis.EMBO J. 1998 Jan 15;17(2):462-9. doi: 10.1093/emboj/17.2.462. EMBO J. 1998. PMID: 9430638 Free PMC article.
-
Strong FtsZ is with the force: mechanisms to constrict bacteria.Trends Microbiol. 2010 Aug;18(8):348-56. doi: 10.1016/j.tim.2010.06.001. Epub 2010 Jul 1. Trends Microbiol. 2010. PMID: 20598544 Review.
-
Mutations in the bacterial cell division protein FtsZ highlight the role of GTP binding and longitudinal subunit interactions in assembly and function.BMC Microbiol. 2015 Oct 13;15:209. doi: 10.1186/s12866-015-0544-z. BMC Microbiol. 2015. PMID: 26463348 Free PMC article.
-
Mutations in ftsZ that confer resistance to SulA affect the interaction of FtsZ with GTP.J Bacteriol. 1994 Jan;176(1):130-6. doi: 10.1128/jb.176.1.130-136.1994. J Bacteriol. 1994. PMID: 8282688 Free PMC article.
-
FtsZDr, a tubulin homologue in radioresistant bacterium Deinococcus radiodurans is characterized as a GTPase exhibiting polymerization/depolymerization dynamics in vitro and FtsZ ring formation in vivo.Int J Biochem Cell Biol. 2014 May;50:38-46. doi: 10.1016/j.biocel.2014.01.015. Epub 2014 Feb 3. Int J Biochem Cell Biol. 2014. PMID: 24502896 Review.
Cited by
-
The Nitrosopumilus maritimus CdvB, but not FtsZ, assembles into polymers.Archaea. 2013;2013:104147. doi: 10.1155/2013/104147. Epub 2013 Jun 2. Archaea. 2013. PMID: 23818813 Free PMC article.
-
Cytokinesis in bacteria.Microbiol Mol Biol Rev. 2003 Mar;67(1):52-65, table of contents. doi: 10.1128/MMBR.67.1.52-65.2003. Microbiol Mol Biol Rev. 2003. PMID: 12626683 Free PMC article. Review.
-
Targeting the Achilles' Heel of Multidrug-Resistant Staphylococcus aureus by the Endocannabinoid Anandamide.Int J Mol Sci. 2022 Jul 14;23(14):7798. doi: 10.3390/ijms23147798. Int J Mol Sci. 2022. PMID: 35887146 Free PMC article.
-
Investigation of regulation of FtsZ assembly by SulA and development of a model for FtsZ polymerization.J Bacteriol. 2008 Apr;190(7):2513-26. doi: 10.1128/JB.01612-07. Epub 2008 Feb 1. J Bacteriol. 2008. PMID: 18245292 Free PMC article.
-
An intrinsically disordered linker plays a critical role in bacterial cell division.Semin Cell Dev Biol. 2015 Jan;37:3-10. doi: 10.1016/j.semcdb.2014.09.017. Epub 2014 Oct 13. Semin Cell Dev Biol. 2015. PMID: 25305578 Free PMC article. Review.
References
-
- Adams E T J, Lewis M S. Sedimentation equilibrium in reacting systems. VI. Some applications to indefinite self-associations. Studies with beta-lactoglobulin A. Biochemistry. 1968;7:1044–1053. - PubMed
-
- Bi E F, Lutkenhaus J. FtsZ ring structure associated with division in Escherichia coli. Nature. 1991;354:161–164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

