Stabilization but not the transcriptional activity of herpes simplex virus VP16-induced complexes is evolutionarily conserved among HCF family members
- PMID: 11711630
- PMCID: PMC116136
- DOI: 10.1128/JVI.75.24.12402-12411.2001
Stabilization but not the transcriptional activity of herpes simplex virus VP16-induced complexes is evolutionarily conserved among HCF family members
Abstract
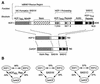
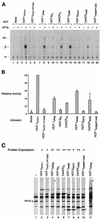
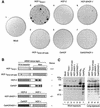
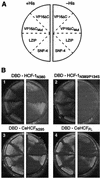
The human herpes simplex virus (HSV) protein VP16 induces formation of a transcriptional regulatory complex with two cellular factors-the POU homeodomain transcription factor Oct-1 and the cell proliferation factor HCF-1-to activate viral immediate-early-gene transcription. Although the cellular role of Oct-1 in transcription is relatively well understood, the cellular role of HCF-1 in cell proliferation is enigmatic. HCF-1 and the related protein HCF-2 form an HCF protein family in humans that is related to a Caenorhabditis elegans homolog called CeHCF. In this study, we show that all three proteins can promote VP16-induced-complex formation, indicating that VP16 targets a highly conserved function of HCF proteins. The resulting VP16-induced complexes, however, display different transcriptional activities. In contrast to HCF-1 and CeHCF, HCF-2 fails to support VP16 activation of transcription effectively. These results suggest that, along with HCF-1, HCF-2 could have a role, albeit probably a different role, in HSV infection. CeHCF can mimic HCF-1 for both association with viral and cellular proteins and transcriptional activation, suggesting that the function(s) of HCF-1 targeted by VP16 has been highly conserved throughout metazoan evolution.
Figures

Similar articles
-
Developmental and cell-cycle regulation of Caenorhabditis elegans HCF phosphorylation.Biochemistry. 2001 May 15;40(19):5786-94. doi: 10.1021/bi010086o. Biochemistry. 2001. PMID: 11341844
-
The herpes simplex virus VP16-induced complex: the makings of a regulatory switch.Trends Biochem Sci. 2003 Jun;28(6):294-304. doi: 10.1016/S0968-0004(03)00088-4. Trends Biochem Sci. 2003. PMID: 12826401 Review.
-
The herpesvirus transactivator VP16 mimics a human basic domain leucine zipper protein, luman, in its interaction with HCF.J Virol. 1998 Aug;72(8):6291-7. doi: 10.1128/JVI.72.8.6291-6297.1998. J Virol. 1998. PMID: 9658067 Free PMC article.
-
Potential role for luman, the cellular homologue of herpes simplex virus VP16 (alpha gene trans-inducing factor), in herpesvirus latency.J Virol. 2000 Jan;74(2):934-43. doi: 10.1128/jvi.74.2.934-943.2000. J Virol. 2000. PMID: 10623756 Free PMC article.
-
Host factors associated with either VP16 or VP16-induced complex differentially affect HSV-1 lytic infection.Rev Med Virol. 2022 Nov;32(6):e2394. doi: 10.1002/rmv.2394. Epub 2022 Sep 7. Rev Med Virol. 2022. PMID: 36069169 Free PMC article. Review.
Cited by
-
Molecular cloning of Drosophila HCF reveals proteolytic processing and self-association of the encoded protein.J Cell Physiol. 2003 Feb;194(2):117-26. doi: 10.1002/jcp.10193. J Cell Physiol. 2003. PMID: 12494450 Free PMC article.
-
The UL14 tegument protein of herpes simplex virus type 1 is required for efficient nuclear transport of the alpha transinducing factor VP16 and viral capsids.J Virol. 2008 Feb;82(3):1094-106. doi: 10.1128/JVI.01226-07. Epub 2007 Nov 21. J Virol. 2008. PMID: 18032514 Free PMC article.
-
Differential subcellular localization and activity of kelch repeat proteins KLHDC1 and KLHDC2.Mol Cell Biochem. 2007 Feb;296(1-2):109-19. doi: 10.1007/s11010-006-9304-6. Epub 2006 Sep 9. Mol Cell Biochem. 2007. PMID: 16964437
-
HCF-2 inhibits cell proliferation and activates differentiation-gene expression programs.Nucleic Acids Res. 2019 Jun 20;47(11):5792-5808. doi: 10.1093/nar/gkz307. Nucleic Acids Res. 2019. PMID: 31049581 Free PMC article.
-
The evolutionarily conserved longevity determinants HCF-1 and SIR-2.1/SIRT1 collaborate to regulate DAF-16/FOXO.PLoS Genet. 2011 Sep;7(9):e1002235. doi: 10.1371/journal.pgen.1002235. Epub 2011 Sep 1. PLoS Genet. 2011. PMID: 21909281 Free PMC article.
References
-
- Adams J, Kelso R, Cooley L. The kelch repeat superfamily of proteins: propellers of cell function. Trends Cell Biol. 2000;10:17–24. - PubMed
-
- Cleary M A, Stern S, Tanaka M, Herr W. Differential positive control by Oct-1 and Oct-2: activation of a transcriptionally silent motif through Oct-1 and VP16 corecruitment. Genes Dev. 1993;7:72–83. - PubMed
-
- Fields S, Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:245–246. - PubMed
-
- Goto H, Motomura S, Wilson A C, Freiman R N, Nakabeppu Y, Fukushima K, Fujishima M, Herr W, Nishimoto T. A single-point mutation in HCF causes temperature-sensitive cell-cycle arrest and disrupts VP16 interaction. Genes Dev. 1997;11:726–732. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

