The N-terminal domain of the t-SNARE Vam3p coordinates priming and docking in yeast vacuole fusion
- PMID: 11694574
- PMCID: PMC60262
- DOI: 10.1091/mbc.12.11.3375
The N-terminal domain of the t-SNARE Vam3p coordinates priming and docking in yeast vacuole fusion
Abstract
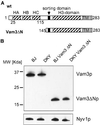
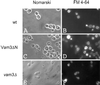
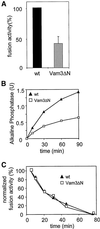
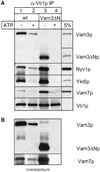
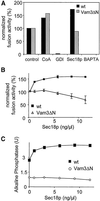
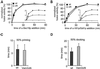
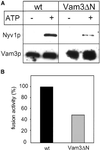
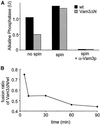
Homotypic fusion of yeast vacuoles requires a regulated sequence of events. During priming, Sec18p disassembles cis-SNARE complexes. The HOPS complex, which is initially associated with the cis-SNARE complex, then mediates tethering. Finally, SNAREs assemble into trans-complexes before the membranes fuse. The t-SNARE of the vacuole, Vam3p, plays a central role in the coordination of these processes. We deleted the N-terminal region of Vam3p to analyze the role of this domain in membrane fusion. The truncated protein (Vam3 Delta N) is sorted normally to the vacuole and is functional, because the vacuolar morphology is unaltered in this strain. However, in vitro vacuole fusion is strongly reduced due to the following reasons: Assembly, as well as disassembly of the cis-SNARE complex is more efficient on Vam3 Delta N vacuoles; however, the HOPS complex is not associated well with the Vam3 Delta N cis-complex. Thus, primed SNAREs from Vam3 Delta N vacuoles cannot participate efficiently in the reaction because trans-SNARE pairing is substantially reduced. We conclude that the N-terminus of Vam3p is required for coordination of priming and docking during homotypic vacuole fusion.
Figures

Similar articles
-
Hierarchy of protein assembly at the vertex ring domain for yeast vacuole docking and fusion.J Cell Biol. 2003 Feb 3;160(3):365-74. doi: 10.1083/jcb.200209095. J Cell Biol. 2003. PMID: 12566429 Free PMC article.
-
Three v-SNAREs and two t-SNAREs, present in a pentameric cis-SNARE complex on isolated vacuoles, are essential for homotypic fusion.J Cell Biol. 1999 Jun 28;145(7):1435-42. doi: 10.1083/jcb.145.7.1435. J Cell Biol. 1999. PMID: 10385523 Free PMC article.
-
A soluble SNARE drives rapid docking, bypassing ATP and Sec17/18p for vacuole fusion.EMBO J. 2004 Jul 21;23(14):2765-76. doi: 10.1038/sj.emboj.7600286. Epub 2004 Jul 8. EMBO J. 2004. PMID: 15241469 Free PMC article.
-
Involvement of LMA1 and GATE-16 family members in intracellular membrane dynamics.Biochim Biophys Acta. 2003 Aug 18;1641(2-3):145-56. doi: 10.1016/s0167-4889(03)00086-7. Biochim Biophys Acta. 2003. PMID: 12914955 Review.
-
Membrane fusion: five lipids, four SNAREs, three chaperones, two nucleotides, and a Rab, all dancing in a ring on yeast vacuoles.Annu Rev Cell Dev Biol. 2010;26:115-36. doi: 10.1146/annurev-cellbio-100109-104131. Annu Rev Cell Dev Biol. 2010. PMID: 20521906 Review.
Cited by
-
Steric hindrance of SNARE transmembrane domain organization impairs the hemifusion-to-fusion transition.EMBO Rep. 2016 Nov;17(11):1590-1608. doi: 10.15252/embr.201642209. Epub 2016 Sep 19. EMBO Rep. 2016. PMID: 27644261 Free PMC article.
-
Structure of the HOPS tethering complex, a lysosomal membrane fusion machinery.Elife. 2022 Sep 13;11:e80901. doi: 10.7554/eLife.80901. Elife. 2022. PMID: 36098503 Free PMC article.
-
New links between vesicle coats and Rab-mediated vesicle targeting.Semin Cell Dev Biol. 2011 Feb;22(1):18-26. doi: 10.1016/j.semcdb.2010.07.003. Epub 2010 Jul 17. Semin Cell Dev Biol. 2011. PMID: 20643221 Free PMC article. Review.
-
SNARE function is not involved in early endosome docking.Mol Biol Cell. 2008 Dec;19(12):5327-37. doi: 10.1091/mbc.e08-05-0457. Epub 2008 Oct 8. Mol Biol Cell. 2008. PMID: 18843044 Free PMC article.
-
The Habc domain of the SNARE Vam3 interacts with the HOPS tethering complex to facilitate vacuole fusion.J Biol Chem. 2015 Feb 27;290(9):5405-13. doi: 10.1074/jbc.M114.631465. Epub 2015 Jan 6. J Biol Chem. 2015. PMID: 25564619 Free PMC article.
References
-
- Chen YA, Scales SJ, Patel SM, Doung YC, Scheller RH. SNARE complex formation is triggered by Ca2+ and drives membrane fusion. Cell. 1999;97:165–174. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

