Recruitment of protein kinase D to the trans-Golgi network via the first cysteine-rich domain
- PMID: 11689438
- PMCID: PMC125696
- DOI: 10.1093/emboj/20.21.5982
Recruitment of protein kinase D to the trans-Golgi network via the first cysteine-rich domain
Abstract
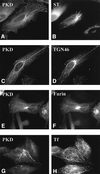
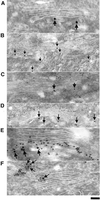
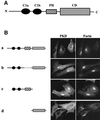
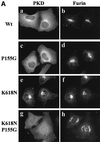
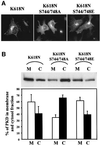
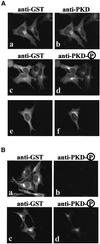
Protein kinase D (PKD) is a cytosolic protein, which upon binding to the trans-Golgi network (TGN) regulates the fission of transport carriers specifically destined to the cell surface. We have found that the first cysteine-rich domain (C1a), but not the second cysteine-rich domain (C1b), is sufficient for the binding of PKD to the TGN. Proline 155 in C1a is necessary for the recruitment of intact PKD to the TGN. Whereas C1a is sufficient to target a reporter protein to the TGN, mutation of serines 744/748 to alanines in the activation loop of intact PKD inhibits its localization to the TGN. Moreover, anti-phospho-PKD antibody, which recognizes only the activated form of PKD, recognizes the TGN-bound PKD. Thus, activation of intact PKD is important for binding to the TGN.
Figures



Similar articles
-
Role of the second cysteine-rich domain and Pro275 in protein kinase D2 interaction with ADP-ribosylation factor 1, trans-Golgi network recruitment, and protein transport.Mol Biol Cell. 2010 Mar 15;21(6):1011-22. doi: 10.1091/mbc.e09-09-0814. Epub 2010 Jan 20. Mol Biol Cell. 2010. PMID: 20089835 Free PMC article.
-
Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane.Science. 2002 Jan 11;295(5553):325-8. doi: 10.1126/science.1066759. Epub 2001 Nov 29. Science. 2002. PMID: 11729268
-
Protein kinase D regulates basolateral membrane protein exit from trans-Golgi network.Nat Cell Biol. 2004 Feb;6(2):106-12. doi: 10.1038/ncb1090. Epub 2004 Jan 25. Nat Cell Biol. 2004. PMID: 14743217 Free PMC article.
-
PKD regulates membrane fission to generate TGN to cell surface transport carriers.Cold Spring Harb Perspect Biol. 2011 Feb 1;3(2):a005280. doi: 10.1101/cshperspect.a005280. Cold Spring Harb Perspect Biol. 2011. PMID: 21421913 Free PMC article. Review.
-
The PKD-Dependent Biogenesis of TGN-to-Plasma Membrane Transport Carriers.Cells. 2021 Jun 28;10(7):1618. doi: 10.3390/cells10071618. Cells. 2021. PMID: 34203456 Free PMC article. Review.
Cited by
-
Architecture of Lipid Droplets in Endoplasmic Reticulum Is Determined by Phospholipid Intrinsic Curvature.Curr Biol. 2018 Mar 19;28(6):915-926.e9. doi: 10.1016/j.cub.2018.02.020. Epub 2018 Mar 8. Curr Biol. 2018. PMID: 29526591 Free PMC article.
-
Global detection of protein kinase D-dependent phosphorylation events in nocodazole-treated human cells.Mol Cell Proteomics. 2012 May;11(5):160-70. doi: 10.1074/mcp.M111.016014. Epub 2012 Apr 10. Mol Cell Proteomics. 2012. PMID: 22496350 Free PMC article.
-
Multifaceted Functions of Protein Kinase D in Pathological Processes and Human Diseases.Biomolecules. 2021 Mar 23;11(3):483. doi: 10.3390/biom11030483. Biomolecules. 2021. PMID: 33807058 Free PMC article. Review.
-
A ubiquitin-like domain controls protein kinase D dimerization and activation by trans-autophosphorylation.J Biol Chem. 2019 Sep 27;294(39):14422-14441. doi: 10.1074/jbc.RA119.008713. Epub 2019 Aug 12. J Biol Chem. 2019. PMID: 31406020 Free PMC article.
-
Regulation of secretory transport by protein kinase D-mediated phosphorylation of the ceramide transfer protein.J Cell Biol. 2007 Jul 2;178(1):15-22. doi: 10.1083/jcb.200612017. Epub 2007 Jun 25. J Cell Biol. 2007. PMID: 17591919 Free PMC article.
References
-
- Dieterich S., Herget,T., Link,G., Bottinger,H., Pfizenmaier,K. and Johannes,F.J. (1996) In vitro activation and substrates of recombinant, baculovirus expressed human protein kinase C µ. FEBS Lett., 381, 183–187. - PubMed
-
- Hausser A., Storz,P., Link,G., Stoll,H., Liu,Y.C., Altman,A., Pfizenmaier,K. and Johannes,F.J. (1999) Protein kinase C µ is negatively regulated by 14-3-3 signal transduction proteins. J. Biol. Chem., 274, 9258–9264. - PubMed
-
- Huijbregts R.P., Topalof,L. and Bankaitis,V.A. (2000) Lipid metabolism and regulation of membrane trafficking. Traffic, 1, 195–202. - PubMed
-
- Iglesias T. and Rozengurt,E. (1998) Protein kinase D activation by mutations within its pleckstrin homology domain. J. Biol. Chem., 273, 410–416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

