Characterization of AFMP1: a novel target for serodiagnosis of aspergillosis
- PMID: 11682494
- PMCID: PMC88451
- DOI: 10.1128/JCM.39.11.3830-3837.2001
Characterization of AFMP1: a novel target for serodiagnosis of aspergillosis
Abstract
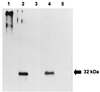
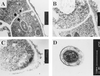
We cloned the AFMP1 gene, which encodes the first antigenic cell wall galactomannoprotein in Aspergillus fumigatus. AFMP1 codes for a protein, Afmp1p, of 284 amino acid residues, with a few sequence features that are present in Mp1p, the antigenic cell wall mannoprotein in Penicillium marneffei that we described previously, as well as several other cell wall proteins of Saccharomyces cerevisiae and Candida albicans. It contains a serine- and threonine-rich region for O glycosylation, a signal peptide, and a putative glycosylphosphatidyl inositol attachment signal sequence. Specific anti-Afmp1p antibody was generated with recombinant Afmp1p protein purified from Escherichia coli to allow further characterization of Afmp1p. Afmp1p has a high affinity for Galanthus nivalis agglutinin, a characteristic indicative of a mannoprotein. Furthermore, it was recognized by a rat monoclonal antibody against the galactofuran side chain of galactomannan, indicating that it is a galactomannoprotein. Ultrastructural analysis by immunogold staining indicated that Afmp1p is present in the cell walls of the hyphae and conidia of A. fumigatus. Finally, it was observed that patients with aspergilloma and invasive aspergillosis due to A. fumigatus develop a specific antibody response against Afmp1p. This suggested that the recombinant protein and its antibody may be useful for serodiagnosis in patients with aspergilloma or invasive aspergillosis, and the protein may represent a good cell surface target for host humoral immunity.
Figures




Similar articles
-
Detection of cell wall galactomannoprotein Afmp1p in culture supernatants of Aspergillus fumigatus and in sera of aspergillosis patients.J Clin Microbiol. 2002 Nov;40(11):4382-7. doi: 10.1128/JCM.40.11.4382-4387.2002. J Clin Microbiol. 2002. PMID: 12409437 Free PMC article.
-
AFMP2 encodes a novel immunogenic protein of the antigenic mannoprotein superfamily in Aspergillus fumigatus.J Clin Microbiol. 2004 May;42(5):2287-91. doi: 10.1128/JCM.42.5.2287-2291.2004. J Clin Microbiol. 2004. PMID: 15131215 Free PMC article.
-
AFLMP1 encodes an antigenic cel wall protein in Aspergillus flavus.J Clin Microbiol. 2003 Feb;41(2):845-50. doi: 10.1128/JCM.41.2.845-850.2003. J Clin Microbiol. 2003. PMID: 12574298 Free PMC article.
-
Cell wall antigens in Aspergillus fumigatus.Arch Med Res. 1993 Autumn;24(3):269-74. Arch Med Res. 1993. PMID: 8298277 Review.
-
Detection of invasive aspergillosis.Adv Appl Microbiol. 2010;70:187-216. doi: 10.1016/S0065-2164(10)70006-X. Epub 2010 Mar 6. Adv Appl Microbiol. 2010. PMID: 20359458 Review.
Cited by
-
Lessons on fruiting body morphogenesis from genomes and transcriptomes of Agaricomycetes.Stud Mycol. 2023 Jul;104:1-85. doi: 10.3114/sim.2022.104.01. Epub 2023 Jan 31. Stud Mycol. 2023. PMID: 37351542 Free PMC article.
-
Antifungal antibodies present in intravenous immunoglobulin derived from China.Braz J Microbiol. 2023 Mar;54(1):81-92. doi: 10.1007/s42770-022-00894-z. Epub 2023 Jan 5. Braz J Microbiol. 2023. PMID: 36602749 Free PMC article.
-
Mini Review: Risk Assessment, Clinical Manifestation, Prediction, and Prognosis of Mucormycosis: Implications for Pathogen- and Human-Derived Biomarkers.Front Microbiol. 2022 Jun 20;13:895989. doi: 10.3389/fmicb.2022.895989. eCollection 2022. Front Microbiol. 2022. PMID: 35794908 Free PMC article. Review.
-
Identifying candidate Aspergillus pathogenicity factors by annotation frequency.BMC Microbiol. 2020 Nov 11;20(1):342. doi: 10.1186/s12866-020-02031-y. BMC Microbiol. 2020. PMID: 33176679 Free PMC article.
-
Aspergillus fumigatus phosphoethanolamine transferase gene gpi7 is required for proper transportation of the cell wall GPI-anchored proteins and polarized growth.Sci Rep. 2019 Apr 10;9(1):5857. doi: 10.1038/s41598-019-42344-1. Sci Rep. 2019. PMID: 30971734 Free PMC article.
References
-
- Caras I W, Weddell G N. Signal peptide for protein secretion directing glycophospholipid membrane anchor attachment. Science. 1989;243:1196–1198. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

