Drosophila MyD88 is an adapter in the Toll signaling pathway
- PMID: 11606776
- PMCID: PMC60109
- DOI: 10.1073/pnas.231471798
Drosophila MyD88 is an adapter in the Toll signaling pathway
Abstract
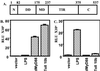
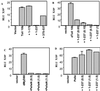
Toll-like receptors comprise a family of cell surface receptors that play a crucial role in the innate immune recognition of both Drosophila and mammals. Previous studies have shown that Drosophila Toll-1 mediates the induction of antifungal peptides during fungal infection of adult flies. Through genetic studies, Tube, Pelle, Cactus, and Dif have been identified as downstream components of the Toll-1 signaling pathway. Here we report characterization of a Drosophila homologue of human MyD88, dMyD88. We show that dMyD88 is an adapter in the Toll signaling pathway that associates with both the Toll receptor and the downstream kinase Pelle. Expression of dMyD88 in S2 cells strongly induced activity of a Drosomycin reporter gene, whereas a dominant-negative version of dMyD88 potently inhibited Toll-mediated signaling. We also show that dMyD88 associates with the death domain-containing adapter Drosophila Fas-associated death domain-containing protein (dFADD), which in turn interacts with the apical caspase Dredd. This pathway links a cell surface receptor to an apical caspase in invertebrate cells and therefore suggests that the Toll-mediated pathway of caspase activation may be the evolutionary ancestor of the death receptor-mediated pathway for apoptosis induction in mammals.
Figures



Similar articles
-
Signaling of apoptosis through TLRs critically involves toll/IL-1 receptor domain-containing adapter inducing IFN-beta, but not MyD88, in bacteria-infected murine macrophages.J Immunol. 2004 Sep 1;173(5):3320-8. doi: 10.4049/jimmunol.173.5.3320. J Immunol. 2004. PMID: 15322195
-
Toll-like receptor 4 and Toll-IL-1 receptor domain-containing adapter protein (TIRAP)/myeloid differentiation protein 88 adapter-like (Mal) contribute to maximal IL-6 expression in macrophages.J Immunol. 2002 Nov 15;169(10):5874-80. doi: 10.4049/jimmunol.169.10.5874. J Immunol. 2002. PMID: 12421970
-
Drosophila MyD88 is required for the response to fungal and Gram-positive bacterial infections.Nat Immunol. 2002 Jan;3(1):91-7. doi: 10.1038/ni747. Epub 2001 Dec 17. Nat Immunol. 2002. PMID: 11743586
-
Toll and Toll-like proteins: an ancient family of receptors signaling infection.Rev Immunogenet. 2000;2(3):294-304. Rev Immunogenet. 2000. PMID: 11256741 Review.
-
Regulation of innate immune responses by Toll-like receptors.Jpn J Infect Dis. 2001 Dec;54(6):209-19. Jpn J Infect Dis. 2001. PMID: 11862002 Review.
Cited by
-
ATF4 is directly recruited by TLR4 signaling and positively regulates TLR4-trigged cytokine production in human monocytes.Cell Mol Immunol. 2013 Jan;10(1):84-94. doi: 10.1038/cmi.2012.57. Epub 2012 Dec 17. Cell Mol Immunol. 2013. PMID: 23241898 Free PMC article.
-
FLICE-like inhibitory protein (FLIP) protects against apoptosis and suppresses NF-kappaB activation induced by bacterial lipopolysaccharide.Am J Pathol. 2004 Oct;165(4):1423-31. doi: 10.1016/s0002-9440(10)63400-1. Am J Pathol. 2004. PMID: 15466406 Free PMC article.
-
Transcriptional responses in honey bee larvae infected with chalkbrood fungus.BMC Genomics. 2010 Jun 21;11:391. doi: 10.1186/1471-2164-11-391. BMC Genomics. 2010. PMID: 20565973 Free PMC article.
-
Tyrosine phosphorylation of MyD88 adapter-like (Mal) is critical for signal transduction and blocked in endotoxin tolerance.J Biol Chem. 2008 Feb 8;283(6):3109-3119. doi: 10.1074/jbc.M707400200. Epub 2007 Dec 10. J Biol Chem. 2008. PMID: 18070880 Free PMC article.
-
Conventional and non-conventional Drosophila Toll signaling.Dev Comp Immunol. 2014 Jan;42(1):16-24. doi: 10.1016/j.dci.2013.04.011. Epub 2013 Apr 28. Dev Comp Immunol. 2014. PMID: 23632253 Free PMC article. Review.
References
-
- Medzhitov R, Janeway C A., Jr Cell. 1997;91:295–298. - PubMed
-
- Anderson K V. Curr Opin Immunol. 2000;12:13–19. - PubMed
-
- Imler J L, Hoffmann J A. Curr Opin Microbiol. 2000;3:16–22. - PubMed
-
- Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway C A., Jr Mol Cell. 1998;2:253–258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

