Exogenous interleukin-2 administration corrects the cell cycle perturbation of lymphocytes from human immunodeficiency virus-infected individuals
- PMID: 11602725
- PMCID: PMC114665
- DOI: 10.1128/JVI.75.22.10843-10855.2001
Exogenous interleukin-2 administration corrects the cell cycle perturbation of lymphocytes from human immunodeficiency virus-infected individuals
Abstract
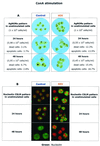
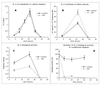
Human immunodeficiency virus (HIV)-induced immunodeficiency is characterized by progressive loss of CD4(+) T cells associated with functional abnormalities of the surviving lymphocytes. Increased susceptibility to apoptosis and loss of proper cell cycle control can be observed in lymphocytes from HIV-infected individuals and may contribute to the lymphocyte dysfunction of AIDS patients. To better understand the relation between T-cell activation, apoptosis, and cell cycle perturbation, we studied the effect of exogenous interleukin-2 (IL-2) administration on the intracellular turnover of phase-dependent proteins. Circulating T cells from HIV-infected patients display a marked discrepancy between a metabolic profile typical of G(0) and a pattern of expression of phase-dependent proteins that indicates a more-advanced position within the cell cycle. This discrepancy is enhanced by in vitro activation with ConA and ultimately results in a marked increase of apoptotic events. Conversely, treatment of lymphocytes with IL-2 alone restores the phase-specific pattern of expression of cell cycle-dependent proteins and is associated with low levels of apoptosis. Interestingly, exogenous IL-2 administration normalizes the overall intracellular protein turnover, as measured by protein synthesis, half-life of newly synthesised proteins, and total protein ubiquitination, thus providing a possible explanation for the effect of IL-2 on the intracellular kinetics of cell cycle-dependent proteins. The beneficial effect of IL-2 administration is consistent with the possibility of defective IL-2 function in vivo, which is confirmed by the observation that lymphocytes from HIV-infected patients show abnormal endogenous IL-2 paracrine/autocrine function upon in vitro mitogen stimulation. Overall these results confirm that perturbation of cell cycle control contributes to HIV-related lymphocyte dysfunction and, by showing that IL-2 administration can revert this perturbation, suggest a new mechanism of action of IL-2 therapy in HIV-infected patients.
Figures





Similar articles
-
Interruption of antiretroviral therapy blunts but does not abrogate CD4 T-cell responses to interleukin-2 administration in HIV infected patients.AIDS. 2006 Feb 14;20(3):361-9. doi: 10.1097/01.aids.0000206502.24407.9f. AIDS. 2006. PMID: 16439869
-
Inconsistent reconstitution of cytomegalovirus-specific cell-mediated immunity in human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy.J Infect Dis. 2001 Sep 15;184(6):707-12. doi: 10.1086/322859. Epub 2001 Jul 30. J Infect Dis. 2001. PMID: 11517431
-
Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with the human immunodeficiency virus.J Clin Invest. 1994 Feb;93(2):768-75. doi: 10.1172/JCI117031. J Clin Invest. 1994. PMID: 8113410 Free PMC article.
-
Cell cycle dysregulation during HIV infection: perspectives of a target based therapy.Curr Drug Targets Immune Endocr Metabol Disord. 2002 Apr;2(1):53-61. Curr Drug Targets Immune Endocr Metabol Disord. 2002. PMID: 12477296 Review.
-
Interleukin-7 (IL-7): immune function, involvement in the pathogenesis of HIV infection and therapeutic potential.Eur Cytokine Netw. 2004 Oct-Dec;15(4):279-89. Eur Cytokine Netw. 2004. PMID: 15627636 Review.
Cited by
-
Perturbations of cell cycle control in T cells contribute to the different outcomes of simian immunodeficiency virus infection in rhesus macaques and sooty mangabeys.J Virol. 2006 Jan;80(2):634-42. doi: 10.1128/JVI.80.2.634-642.2006. J Virol. 2006. PMID: 16378966 Free PMC article.
-
Dysregulation of the polo-like kinase pathway in CD4+ T cells is characteristic of pathogenic simian immunodeficiency virus infection.J Virol. 2004 Feb;78(3):1464-72. doi: 10.1128/jvi.78.3.1464-1472.2004. J Virol. 2004. PMID: 14722302 Free PMC article.
-
Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells.J Exp Med. 2004 Sep 20;200(6):701-12. doi: 10.1084/jem.20041270. J Exp Med. 2004. PMID: 15381726 Free PMC article.
-
RNA viruses: hijacking the dynamic nucleolus.Nat Rev Microbiol. 2007 Feb;5(2):119-27. doi: 10.1038/nrmicro1597. Nat Rev Microbiol. 2007. PMID: 17224921 Free PMC article. Review.
-
The cell cycle and virus infection.Methods Mol Biol. 2005;296:197-218. doi: 10.1385/1-59259-857-9:197. Methods Mol Biol. 2005. PMID: 15576934 Free PMC article.
References
-
- Adachi Y, Oyaizu N, Than S, McCloskey T W, Pahwa S. IL-2 rescues in vitro lymphocyte apoptosis in patients with HIV infection: correlation with its ability to block culture-induced down-modulation of bcl-2. J Immunol. 1996;157:4184–4193. - PubMed
-
- Amendola A, Poccia F, Martini F, Gioia C, Galati V, Pierdominici M, Marziali M, Pandolfi F, Colizzi V, Piacentini M, Girardi E, D'offizi G. Decreased CD95 expression on naive T cells from HIV-infected persons undergoing highly active anti-retroviral therapy (HAART) and the influence of IL-2 low dose administration. Irhan Group Study. Clin Exp Immunol. 2000;120:324–332. - PMC - PubMed
-
- Baserga R. Oncogenes and the strategy of growth factors. Cell. 1994;79:927–930. - PubMed
-
- Bohler T, Walcher J, Holzl-Wenig G, Geiss M, Buchholz B, Linde R, Debatin K M. Early effects of antiretroviral combination therapy on activation, apoptosis and regeneration of T cells in HIV-1-infected children and adolescents. AIDS. 1999;13:779–789. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

