Dendritic cell maturation overrules H-2D-mediated natural killer T (NKT) cell inhibition: critical role for B7 in CD1d-dependent NKT cell interferon gamma production
- PMID: 11602646
- PMCID: PMC2193518
- DOI: 10.1084/jem.194.8.1179
Dendritic cell maturation overrules H-2D-mediated natural killer T (NKT) cell inhibition: critical role for B7 in CD1d-dependent NKT cell interferon gamma production
Erratum in
- J Exp Med 2001 Nov 5;194(9):1391
Abstract
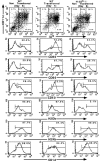
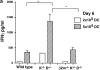
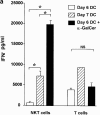
Given the broad expression of H-2 class Ib molecules on hematopoietic cells, antigen presentation pathways among CD1d expressing cells might tightly regulate CD1d-restricted natural killer T (NKT) cells. Bone marrow-derived dendritic cells (BM-DCs) and not adherent splenocytes become capable of triggering NK1.1(+)/T cell receptor (TCR)(int) hepatic NKT cell activation when (a) immature BM-DCs lack H-2D(b)-/- molecules or (b) BM-DCs undergo a stress signal of activation. In such conditions, BM-DCs promote T helper type 1 predominant CD1d-restricted NKT cell stimulation. H-2 class Ia-mediated inhibition involves more the direct H-2D(b) presentation than the indirect Qa-1(b) pathway. Such inhibition can be overruled by B7/CD28 interactions and marginally by CD40/CD40L or interleukin 12. These data point to a unique regulatory role of DCs in NKT cell innate immune responses and suggest that H-2 class Ia and Ib pathways differentially control NKT cell recognition of DC antigens.
Figures






Similar articles
-
Resistance to malarial infection is achieved by the cooperation of NK1.1(+) and NK1.1(-) subsets of intermediate TCR cells which are constituents of innate immunity.Cell Immunol. 2001 Aug 1;211(2):96-104. doi: 10.1006/cimm.2001.1833. Cell Immunol. 2001. PMID: 11591113
-
A subset of NKT cells that lacks the NK1.1 marker, expresses CD1d molecules, and autopresents the alpha-galactosylceramide antigen.J Immunol. 2000 Nov 1;165(9):4917-26. doi: 10.4049/jimmunol.165.9.4917. J Immunol. 2000. PMID: 11046017
-
Human invariant V alpha 24-J alpha Q TCR supports the development of CD1d-dependent NK1.1+ and NK1.1- T cells in transgenic mice.J Immunol. 2003 Mar 1;170(5):2390-8. doi: 10.4049/jimmunol.170.5.2390. J Immunol. 2003. PMID: 12594262
-
Regulation of immune responses by CD1d-restricted natural killer T cells.Immunol Res. 2004;30(2):139-53. doi: 10.1385/IR:30:2:139. Immunol Res. 2004. PMID: 15477656 Review.
-
Innate Valpha14(+) natural killer T cells mature dendritic cells, leading to strong adaptive immunity.Immunol Rev. 2007 Dec;220:183-98. doi: 10.1111/j.1600-065X.2007.00561.x. Immunol Rev. 2007. PMID: 17979847 Review.
Cited by
-
Innate self recognition by an invariant, rearranged T-cell receptor and its immune consequences.Immunology. 2003 Jun;109(2):171-84. doi: 10.1046/j.1365-2567.2003.01657.x. Immunology. 2003. PMID: 12757612 Free PMC article. Review.
-
CD1d-restricted T cells regulate dendritic cell function and antitumor immunity in a granulocyte-macrophage colony-stimulating factor-dependent fashion.Proc Natl Acad Sci U S A. 2003 Jul 22;100(15):8874-9. doi: 10.1073/pnas.1033098100. Epub 2003 Jul 7. Proc Natl Acad Sci U S A. 2003. PMID: 12847287 Free PMC article.
-
IFN-gamma-mediated negative feedback regulation of NKT-cell function by CD94/NKG2.Blood. 2005 Jul 1;106(1):184-92. doi: 10.1182/blood-2004-11-4257. Epub 2005 Mar 3. Blood. 2005. PMID: 15746081 Free PMC article.
-
NKT cell stimulation with glycolipid antigen in vivo: costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge.J Immunol. 2005 Sep 1;175(5):3092-3101. doi: 10.4049/jimmunol.175.5.3092. J Immunol. 2005. PMID: 16116198 Free PMC article.
-
Demonstration of the Antitumor Activity of the iNKT Agonist ABX196, a Novel Enhancer of Cancer Immunotherapy, in Melanoma and Hepatocarcinoma Mouse Models.Mol Cancer Ther. 2022 Dec 2;21(12):1788-1797. doi: 10.1158/1535-7163.MCT-22-0183. Mol Cancer Ther. 2022. PMID: 36198025 Free PMC article.
References
-
- Bendelac A., Riversa M.N., Park S.H., Roark J.H. Mouse CD1-specific NK1+ T cellsdevelopment, specificity, and function. Annu. Rev. Immunol. 1997;15:535–562. - PubMed
-
- Kawano T., Cui J., Koezuka Y., Toura I., Kaneko Y., Motoki K., Ueno H., Nakagawa R., Sato H., Kondo E. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. - PubMed
-
- Burdin N., Brossay L., Koezuka Y., Smily S.T., Gurusby M., Gui M., Taniguchi M., Hayakawa K., Kronenberg M. Selective ability of mouse CD1 to present glycolipids. α-galactosylceramide specifically stimulates Vα14 NKT lymphocytes. J. Immunol. 1998;161:3271–3281. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

