Influence of trehalose on the molecular chaperone activity of p26, a small heat shock/alpha-crystallin protein
- PMID: 11599574
- PMCID: PMC434390
- DOI: 10.1379/1466-1268(2001)006<0126:iototm>2.0.co;2
Influence of trehalose on the molecular chaperone activity of p26, a small heat shock/alpha-crystallin protein
Abstract
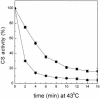
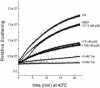
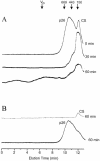
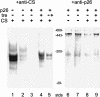
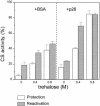
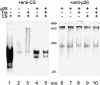
Encysted embryos of the primitive crustacean Artemia franciscana are among the most resistant of all multicellular eukaryotes to environmental stress, in part due to massive amounts of a small heat shock/alpha-crystallin protein (p26) that acts as a molecular chaperone. These embryos also contain very large amounts of the disaccharide trehalose, well known for its ability to protect macromolecules and membranes against damage due to water removal and temperature extremes. Therefore, we looked for potential interactions between trehalose and p26 in the protection of a model substrate, citrate synthase (CS), against heat denaturation and aggregation and in the restoration of activity after heating in vitro. Both trehalose and p26 decreased the aggregation and irreversible inactivation of CS at 43 degrees C. At approximate physiological concentrations (0.4 M), trehalose did not interfere with the ability of p26 to assist in the reactivation of CS after heating, but higher concentrations (0.8 M) were inhibitory. We also showed that CS and p26 interact physically during heating and that trehalose interferes with complex formation and disrupts CS-p26 complexes that form at high temperatures. We suggest from these results that trehalose may act as a "release factor," freeing folding intermediates of CS that p26 can chaperone to the native state. Trehalose and p26 can act synergistically in vitro, during and after thermal stress, suggesting that these interactions also occur in vivo.
Figures
Similar articles
-
A small heat shock/alpha-crystallin protein from encysted Artemia embryos suppresses tubulin denaturation.Cell Stress Chaperones. 2003 Summer;8(2):183-93. doi: 10.1379/1466-1268(2003)008<0183:ashcpf>2.0.co;2. Cell Stress Chaperones. 2003. PMID: 14627204 Free PMC article.
-
Purification, structure and in vitro molecular-chaperone activity of Artemia p26, a small heat-shock/alpha-crystallin protein.Eur J Biochem. 1997 Jan 15;243(1-2):225-32. doi: 10.1111/j.1432-1033.1997.0225a.x. Eur J Biochem. 1997. PMID: 9030743
-
A small heat-shock protein, p26, from the crustacean Artemia protects mammalian cells (Cos-1) against oxidative damage.Cell Biol Int. 2004;28(6):449-55. doi: 10.1016/j.cellbi.2004.03.014. Cell Biol Int. 2004. PMID: 15223021
-
Molecular chaperones, stress resistance and development in Artemia franciscana.Semin Cell Dev Biol. 2003 Oct;14(5):251-8. doi: 10.1016/j.semcdb.2003.09.019. Semin Cell Dev Biol. 2003. PMID: 14986854 Review.
-
Chaperone-like activity of alpha-crystallin and other small heat shock proteins.Curr Protein Pept Sci. 2001 Sep;2(3):205-25. doi: 10.2174/1389203013381107. Curr Protein Pept Sci. 2001. PMID: 12369933 Review.
Cited by
-
The use of trehalose in the preparation of specimens for molecular electron microscopy.Micron. 2011 Dec;42(8):762-72. doi: 10.1016/j.micron.2011.06.005. Epub 2011 Jun 25. Micron. 2011. PMID: 21752659 Free PMC article. Review.
-
Deubiquitinating enzyme BAP1 is involved in the formation and maintenance of the diapause embryos of Artemia.Cell Stress Chaperones. 2012 Sep;17(5):577-87. doi: 10.1007/s12192-012-0333-7. Epub 2012 Feb 29. Cell Stress Chaperones. 2012. PMID: 22374320 Free PMC article.
-
LEA proteins prevent protein aggregation due to water stress.Biochem J. 2005 May 15;388(Pt 1):151-7. doi: 10.1042/BJ20041931. Biochem J. 2005. PMID: 15631617 Free PMC article.
-
A small heat shock/alpha-crystallin protein from encysted Artemia embryos suppresses tubulin denaturation.Cell Stress Chaperones. 2003 Summer;8(2):183-93. doi: 10.1379/1466-1268(2003)008<0183:ashcpf>2.0.co;2. Cell Stress Chaperones. 2003. PMID: 14627204 Free PMC article.
-
Long-term cold acclimation extends survival time at 0°C and modifies the metabolomic profiles of the larvae of the fruit fly Drosophila melanogaster.PLoS One. 2011;6(9):e25025. doi: 10.1371/journal.pone.0025025. Epub 2011 Sep 21. PLoS One. 2011. PMID: 21957472 Free PMC article.
References
-
- Allison SD, Chang B, Randolph TW, Carpenter JF. Hydrogen bonding between sugar and protein is responsible for inhibition of dehydration-induced protein unfolding. Arch Biochem Biophys. 1999;365:289–298. - PubMed
-
- Alexandre H, Plourde L, Charpentier C, Francois J. Lack of correlation between trehalose accumulation, cell viability and intracellular acidification as induced by various stresses in Saccharomyces cerevisiae. Microbiology. 1998;144:1103–1111. - PubMed
-
- Arrigo A-P, Landry J 1994 Expression and function of the low-molecular-weight heat shock proteins. In: The Biology of Heat Shock Proteins and Molecular Chaperones, ed Morimoto RI, Tissieres A, Georgopoulos C. Cold Spring Harbor Laboratory Press, New York, 335–373.
-
- Beissinger M, Buchner J. How chaperones fold proteins. Biol Chem. 1998;379:245–259. - PubMed
-
- Browne RA, Sorgeloos P, and Trotman CNA 1991 Artemia Biology. CRC Press, Boca Raton, FL.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
