PKCbeta modulates antigen receptor signaling via regulation of Btk membrane localization
- PMID: 11598012
- PMCID: PMC125669
- DOI: 10.1093/emboj/20.20.5692
PKCbeta modulates antigen receptor signaling via regulation of Btk membrane localization
Abstract
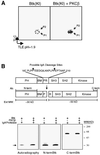
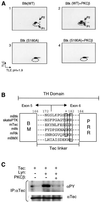
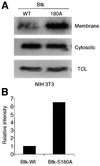
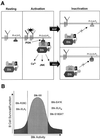
Mutations in Bruton's tyrosine kinase (Btk) result in X-linked agammaglobulinemia (XLA) in humans and X-linked immunodeficiency (xid) in mice. While targeted disruption of the protein kinase C-beta (PKCbeta) gene in mice results in an immunodeficiency similar to xid, the overall tyrosine phosphorylation of Btk is significantly enhanced in PKCbeta-deficient B cells. We provide direct evidence that PKCbeta acts as a feedback loop inhibitor of Btk activation. Inhibition of PKCbeta results in a dramatic increase in B-cell receptor (BCR)-mediated Ca2+ signaling. We identified a highly conserved PKCbeta serine phosphorylation site in a short linker within the Tec homology domain of Btk. Mutation of this phosphorylation site led to enhanced tyrosine phosphorylation and membrane association of Btk, and augmented BCR and FcepsilonRI-mediated signaling in B and mast cells, respectively. These findings provide a novel mechanism whereby reversible translocation of Btk/Tec kinases regulates the threshold for immunoreceptor signaling and thereby modulates lymphocyte activation.
Figures



Similar articles
-
Phosphorylation of two regulatory tyrosine residues in the activation of Bruton's tyrosine kinase via alternative receptors.Proc Natl Acad Sci U S A. 1997 Oct 14;94(21):11526-33. doi: 10.1073/pnas.94.21.11526. Proc Natl Acad Sci U S A. 1997. PMID: 9326643 Free PMC article.
-
Phosphorylation of CD19 Y484 and Y515, and linked activation of phosphatidylinositol 3-kinase, are required for B cell antigen receptor-mediated activation of Bruton's tyrosine kinase.J Immunol. 1999 Apr 15;162(8):4438-46. J Immunol. 1999. PMID: 10201980
-
Selective role of PKCbeta enzymatic function in regulating cell survival mediated by B cell antigen receptor cross-linking.Immunol Lett. 2006 May 15;105(1):83-9. doi: 10.1016/j.imlet.2006.01.006. Epub 2006 Feb 20. Immunol Lett. 2006. PMID: 16564096
-
Functions of Bruton's tyrosine kinase in mast and B cells.J Leukoc Biol. 1999 Mar;65(3):286-90. doi: 10.1002/jlb.65.3.286. J Leukoc Biol. 1999. PMID: 10080529 Review.
-
Regulation of B lymphocyte development and activation by Bruton's tyrosine kinase.Immunol Res. 2001;23(2-3):147-56. doi: 10.1385/IR:23:2-3:147. Immunol Res. 2001. PMID: 11444380 Review.
Cited by
-
A novel transgenic mouse strain expressing PKCβII demonstrates expansion of B1 and marginal zone B cell populations.Sci Rep. 2020 Aug 4;10(1):13156. doi: 10.1038/s41598-020-70191-y. Sci Rep. 2020. PMID: 32753714 Free PMC article.
-
Genetics of primary sclerosing cholangitis and pathophysiological implications.Nat Rev Gastroenterol Hepatol. 2017 May;14(5):279-295. doi: 10.1038/nrgastro.2016.154. Epub 2017 Mar 15. Nat Rev Gastroenterol Hepatol. 2017. PMID: 28293027 Review.
-
Protein kinase C beta controls nuclear factor kappaB activation in B cells through selective regulation of the IkappaB kinase alpha.J Exp Med. 2002 Jun 17;195(12):1647-52. doi: 10.1084/jem.20020408. J Exp Med. 2002. PMID: 12070292 Free PMC article.
-
Differential protein-protein interactions underlie signaling mediated by the TCR and a 4-1BB domain-containing CAR.Sci Signal. 2024 Mar 5;17(826):eadd4671. doi: 10.1126/scisignal.add4671. Epub 2024 Mar 5. Sci Signal. 2024. PMID: 38442200 Free PMC article.
-
A novel oncogenic BTK isoform is overexpressed in colon cancers and required for RAS-mediated transformation.Oncogene. 2016 Aug 18;35(33):4368-78. doi: 10.1038/onc.2015.504. Epub 2016 Jan 25. Oncogene. 2016. PMID: 26804170 Free PMC article.
References
-
- Andreotti A.H., Bunnell,S.C., Feng,S., Berg,L.J. and Schreiber,S.L. (1997) Regulatory intramolecular association in a tyrosine kinase of the Tec family. Nature, 385, 93–97. - PubMed
-
- Baraldi E., Carugo,K.D., Hyvonen,M., Surdo,P.L., Riley,A.M., Potter,B.V., O’Brien,R., Ladbury,J.E. and Saraste,M. (1999) Structure of the PH domain from Bruton’s tyrosine kinase in complex with inositol 1,3,4,5-tetrakisphosphate. Structure, 7, 449–460. - PubMed
-
- Bolland S., Pearse,R.N., Kurosaki,T. and Ravetch,J.V. (1998) SHIP modulates immune receptor responses by regulating membrane association of Btk. Immunity, 8, 509–516. - PubMed
-
- Buhl A.M. and Cambier,J.C. (1997) Co-receptor and accessory regulation of B-cell antigen receptor signal transduction. Immunol. Rev., 160, 127–138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

