TRAF6 is a critical mediator of signal transduction by the viral oncogene latent membrane protein 1
- PMID: 11598011
- PMCID: PMC125680
- DOI: 10.1093/emboj/20.20.5678
TRAF6 is a critical mediator of signal transduction by the viral oncogene latent membrane protein 1
Abstract
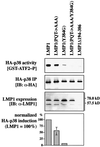
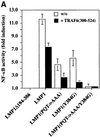
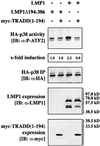
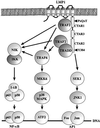
The oncogenic latent membrane protein 1 (LMP1) of the Epstein-Barr virus recruits tumor necrosis factor-receptor (TNFR)-associated factors (TRAFs), the TNFR-associated death domain protein (TRADD) and JAK3 to induce intracellular signaling pathways. LMP1 serves as the prototype of a TRADD-binding receptor that transforms cells but does not induce apoptosis. Here we show that TRAF6 critically mediates LMP1 signaling to p38 mitogen-activated protein kinase (MAPK) via a MAPK kinase 6-dependent pathway. In addition, NF-kappaB but not c-Jun N-terminal kinase 1 (JNK1) induction by LMP1 involves TRAF6. The PxQxT motif of the LMP1 C-terminal activator region 1 (CTAR1) and tyrosine 384 of CTAR2 together are essential for full p38 MAPK activation and for TRAF6 recruitment to the LMP1 signaling complex. Dominant-negative TRADD blocks p38 MAPK activation by LMP1. The data suggest that entry of TRAF6 into the LMP1 complex is mediated by TRADD and TRAF2. In TRAF6-knockout fibroblasts, significant induction of p38 MAPK by LMP1 is dependent on the ectopic expression of TRAF6. We describe a novel role of TRAF6 as an essential signaling mediator of a transforming oncogene, downstream of TRADD and TRAF2.
Figures










Similar articles
-
Epstein-Barr virus-encoded latent membrane protein 1 activates the JNK pathway through its extreme C terminus via a mechanism involving TRADD and TRAF2.J Virol. 1999 Feb;73(2):1023-35. doi: 10.1128/JVI.73.2.1023-1035.1999. J Virol. 1999. PMID: 9882303 Free PMC article.
-
LMP1 signal transduction differs substantially from TNF receptor 1 signaling in the molecular functions of TRADD and TRAF2.EMBO J. 1999 May 4;18(9):2511-21. doi: 10.1093/emboj/18.9.2511. EMBO J. 1999. PMID: 10228165 Free PMC article.
-
Unique signaling properties of CTAR1 in LMP1-mediated transformation.J Virol. 2007 Sep;81(18):9680-92. doi: 10.1128/JVI.01001-07. Epub 2007 Jul 11. J Virol. 2007. PMID: 17626074 Free PMC article.
-
LMP1 TRAFficking activates growth and survival pathways.Adv Exp Med Biol. 2007;597:173-87. doi: 10.1007/978-0-387-70630-6_14. Adv Exp Med Biol. 2007. PMID: 17633026 Review.
-
Pursuing different 'TRADDes': TRADD signaling induced by TNF-receptor 1 and the Epstein-Barr virus oncoprotein LMP1.Biol Chem. 2008 Oct;389(10):1261-71. doi: 10.1515/BC.2008.144. Biol Chem. 2008. PMID: 18713013 Review.
Cited by
-
Epstein-Barr virus deubiquitinase downregulates TRAF6-mediated NF-κB signaling during productive replication.J Virol. 2013 Apr;87(7):4060-70. doi: 10.1128/JVI.02020-12. Epub 2013 Jan 30. J Virol. 2013. PMID: 23365429 Free PMC article.
-
NF-κB Pathways in the Pathogenesis of Multiple Sclerosis and the Therapeutic Implications.Front Mol Neurosci. 2016 Sep 15;9:84. doi: 10.3389/fnmol.2016.00084. eCollection 2016. Front Mol Neurosci. 2016. PMID: 27695399 Free PMC article. Review.
-
Epstein-Barr virus latent membrane protein 1 activation of NF-kappaB through IRAK1 and TRAF6.Proc Natl Acad Sci U S A. 2003 Dec 23;100(26):15595-600. doi: 10.1073/pnas.2136756100. Epub 2003 Dec 12. Proc Natl Acad Sci U S A. 2003. PMID: 14673102 Free PMC article.
-
The Central Role of the Ubiquitin-Proteasome System in EBV-Mediated Oncogenesis.Cancers (Basel). 2022 Jan 26;14(3):611. doi: 10.3390/cancers14030611. Cancers (Basel). 2022. PMID: 35158879 Free PMC article. Review.
-
E3 Ubiquitin Ligases in Gammaherpesviruses and HIV: A Review of Virus Adaptation and Exploitation.Viruses. 2023 Sep 15;15(9):1935. doi: 10.3390/v15091935. Viruses. 2023. PMID: 37766341 Free PMC article. Review.
References
-
- Aicher A., Shu,G.L., Magaletti,D., Mulvania,T., Pezzutto,A., Craxton,A. and Clark,E.A. (1999) Differential role for p38 mitogen-activated protein kinase in regulating CD40-induced gene expression in dendritic cells and B cells. J. Immunol., 163, 5786–5795. - PubMed
-
- Arch R.H., Gedrich,R.W. and Thompson,C.B. (1998) Tumor necrosis factor receptor-associated factors (TRAFs) — a family of adapter proteins that regulates life and death. Genes Dev., 12, 2821–2830. - PubMed
-
- Aviel S., Winberg,G., Massucci,M. and Ciechanover,A. (2000) Degradation of the Epstein–Barr virus latent membrane protein 1 (LMP1) by the ubiquitin-proteasome pathway. Targeting via ubiquitination of the N-terminal residue. J. Biol. Chem., 275, 23491–23499. - PubMed
-
- Baichwal V.R. and Sugden,B. (1988) Transformation of Balb 3T3 cells by the BNLF-1 gene of Epstein–Barr virus. Oncogene, 2, 461–467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

