Hemagglutinin 1-specific immunoglobulin G and Fab molecules mediate postattachment neutralization of influenza A virus by inhibition of an early fusion event
- PMID: 11581389
- PMCID: PMC114595
- DOI: 10.1128/JVI.75.21.10208-10218.2001
Hemagglutinin 1-specific immunoglobulin G and Fab molecules mediate postattachment neutralization of influenza A virus by inhibition of an early fusion event
Abstract
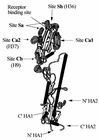
In standard neutralization (STAN), virus and antibody are reacted together before inoculation of target cells, and inhibition of almost any of the processes concerned in the early interaction of virus and cell, including inhibition of virus attachment to cell receptors, can be the cause of neutralization by a particular monoclonal antibody (MAb). To simplify the interpretation of antibody action, we carried out a study of postattachment neutralization (PAN), where virus is allowed to attach to target cells before neutralizing antibody is introduced. We used influenza virus A/PR/8/34 (H1N1) and monoclonal immunoglobulin G (IgG) molecules and their Fabs specific to antigenic sites Sb (tip), Ca2 (loop), and Cb (hinge) of the hemagglutinin 1 (HA1) protein. All IgGs and Fabs gave PAN, although with reduced efficiency compared with STAN. Thus, bivalent binding of antibody was not essential for PAN. By definition, none of these MAbs gave PAN by inhibiting virus attachment, and they did not elute attached virus from the target cell or inhibit endocytosis of virus. However, virus-cell fusion, as demonstrated by R18 fluorescence dequenching or hemolysis of red blood cells, was inhibited in direct proportion to neutralization and in a dose-dependent manner and was thus likely to be responsible for the observed neutralization. However, to get PAN, it was necessary to inhibit the activation of the prefusion intermediate, the earliest known form on the fusion pathway that is created when virus is incubated at pH 5 and 4 degrees C. PAN antibodies may act by binding HA trimers in contact with the cell and/or trimers in the immediate vicinity of the virus-cell contact point and so inhibit the recruitment of additional receptor-HA complexes.
Figures

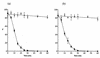
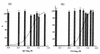
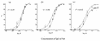

Similar articles
-
Two influenza A virus-specific Fabs neutralize by inhibiting virus attachment to target cells, while neutralization by their IgGs is complex and occurs simultaneously through fusion inhibition and attachment inhibition.Virology. 2000 Dec 20;278(2):423-35. doi: 10.1006/viro.2000.0631. Virology. 2000. PMID: 11118365
-
IgG neutralization of type A influenza viruses and the inhibition of the endosomal fusion stage of the infectious pathway in BHK cells.Virology. 1993 Aug;195(2):413-21. doi: 10.1006/viro.1993.1391. Virology. 1993. PMID: 8337821
-
Variations in the neutralizing and haemagglutination-inhibiting activities of five influenza A virus-specific IgGs and their antibody fragments.J Gen Virol. 1997 Oct;78 ( Pt 10):2431-9. doi: 10.1099/0022-1317-78-10-2431. J Gen Virol. 1997. PMID: 9349461
-
Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin.Annu Rev Biochem. 2000;69:531-69. doi: 10.1146/annurev.biochem.69.1.531. Annu Rev Biochem. 2000. PMID: 10966468 Review.
-
Haemagglutination-inhibiting antibody to influenza virus.Dev Biol (Basel). 2003;115:63-73. Dev Biol (Basel). 2003. PMID: 15088777 Review.
Cited by
-
Distinct mechanisms of neutralization by monoclonal antibodies specific for sites in the N-terminal or C-terminal domain of murine leukemia virus SU.J Virol. 2003 Apr;77(7):3993-4003. doi: 10.1128/jvi.77.7.3993-4003.2003. J Virol. 2003. PMID: 12634359 Free PMC article.
-
The Density Code for the Development of a Vaccine?J Pharm Sci. 2016 Nov;105(11):3223-3232. doi: 10.1016/j.xphs.2016.07.020. Epub 2016 Sep 17. J Pharm Sci. 2016. PMID: 27649885 Free PMC article. Review.
-
Monoclonal antibodies for prophylactic and therapeutic use against viral infections.Pediatr Pol. 2013 Sep-Oct;88(5):T15-T23. doi: 10.1016/j.pepo.2013.08.006. Epub 2013 Aug 23. Pediatr Pol. 2013. PMID: 32287402 Free PMC article. Review.
-
Unraveling of a neutralization mechanism by two human antibodies against conserved epitopes in the globular head of H5 hemagglutinin.J Virol. 2013 Mar;87(6):3571-7. doi: 10.1128/JVI.01292-12. Epub 2012 Dec 26. J Virol. 2013. PMID: 23269809 Free PMC article.
-
A novel humanized antibody neutralizes H5N1 influenza virus via two different mechanisms.J Virol. 2015 Apr;89(7):3712-22. doi: 10.1128/JVI.03014-14. Epub 2015 Jan 21. J Virol. 2015. PMID: 25609802 Free PMC article.
References
-
- Arendrup M, Sönnerborg A, Svennerholm B, Åkerblom L, Nielsen C, Clausen H, Olofsson S, Nielsen J O, Hansen J-E S. Neutralizing antibody response during human immunodeficiency virus type 1 infection: type and group specificity and viral escape. J Gen Virol. 1993;74:855–863. - PubMed
-
- Armstrong S J, Dimmock N J. Varying temperature-dependence of post-attachment neutralization of human immunodeficiency virus type 1 by monoclonal antibodies to gp120: identification of a very early fusion-independent event as a neutralization target. J Gen Virol. 1996;77:1397–1402. - PubMed
-
- Armstrong S J, McInerney T L, McLain L, Wahren B, Hinkula J, Levi M, Dimmock N J. Two neutralizing anti-V3 monoclonal antibodies act by affecting different functions of human immunodeficiency virus type 1. J Gen Virol. 1996;77:2931–2941. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

