Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia
- PMID: 11567062
- PMCID: PMC6762894
- DOI: 10.1523/JNEUROSCI.21-19-07724.2001
Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia
Abstract
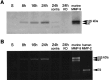
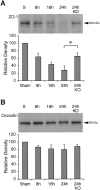
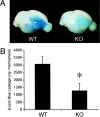
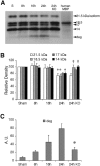
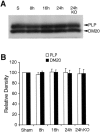
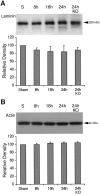
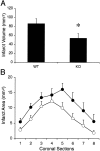
Deleterious processes of extracellular proteolysis may contribute to the progression of tissue damage after acute brain injury. We recently showed that matrix metalloproteinase-9 (MMP-9) knock-out mice were protected against ischemic and traumatic brain injury. In this study, we examined the mechanisms involved by focusing on relevant MMP-9 substrates in blood-brain barrier, matrix, and white matter. MMP-9 knock-out and wild-type mice were subjected to transient focal ischemia. MMP-9 levels increased after ischemia in wild-type brain, with expression primarily present in vascular endothelium. Western blots showed that the blood-brain barrier-associated protein and MMP-9 substrate zonae occludens-1 was degraded after ischemia, but this was reduced in knock-out mice. There were no detectable changes in another blood-brain barrier-associated protein, occludin. Correspondingly, blood-brain barrier disruption assessed via Evans Blue leakage was significantly attenuated in MMP-9 knock-out mice compared with wild types. In white matter, ischemic degradation of the MMP-9 substrate myelin basic protein was significantly reduced in knock-out mice compared with wild types, whereas there was no degradation of other myelin proteins that are not MMP substrates (proteolipid protein and DM20). There were no detectable changes in the ubiquitous structural protein actin or the extracellular matrix protein laminin. Finally, 24 hr lesion volumes were significantly reduced in knock-out mice compared with wild types. These data demonstrate that the protective effects of MMP-9 gene knock-out after transient focal ischemia may be mediated by reduced proteolytic degradation of critical blood-brain barrier and white matter components.
Figures

Similar articles
-
Matrix metalloproteinase-9 gene knock-out protects the immature brain after cerebral hypoxia-ischemia.J Neurosci. 2007 Feb 14;27(7):1511-8. doi: 10.1523/JNEUROSCI.4391-06.2007. J Neurosci. 2007. PMID: 17301159 Free PMC article.
-
Normobaric hyperoxia attenuates early blood-brain barrier disruption by inhibiting MMP-9-mediated occludin degradation in focal cerebral ischemia.J Neurochem. 2009 Feb;108(3):811-20. doi: 10.1111/j.1471-4159.2008.05821.x. J Neurochem. 2009. PMID: 19187098 Free PMC article.
-
Early appearance of activated matrix metalloproteinase-9 and blood-brain barrier disruption in mice after focal cerebral ischemia and reperfusion.Brain Res. 1999 Sep 18;842(1):92-100. doi: 10.1016/s0006-8993(99)01843-0. Brain Res. 1999. PMID: 10526099
-
The role of hypoxia-inducible factor-1α, aquaporin-4, and matrix metalloproteinase-9 in blood-brain barrier disruption and brain edema after traumatic brain injury.J Neurosurg. 2011 Jan;114(1):92-101. doi: 10.3171/2010.6.JNS10207. Epub 2010 Jul 9. J Neurosurg. 2011. PMID: 20617879
-
Matrix metalloproteinase-9 deficiency leads to prolonged foreign body response in the brain associated with increased IL-1beta levels and leakage of the blood-brain barrier.Matrix Biol. 2009 Apr;28(3):148-59. doi: 10.1016/j.matbio.2009.02.002. Epub 2009 Mar 3. Matrix Biol. 2009. PMID: 19264129 Free PMC article.
Cited by
-
β1 integrin-focal adhesion kinase (FAK) signaling modulates retinal ganglion cell (RGC) survival.PLoS One. 2012;7(10):e48332. doi: 10.1371/journal.pone.0048332. Epub 2012 Oct 31. PLoS One. 2012. PMID: 23118988 Free PMC article.
-
TIMP1 preserves the blood-brain barrier through interacting with CD63/integrin β 1 complex and regulating downstream FAK/RhoA signaling.Acta Pharm Sin B. 2020 Jun;10(6):987-1003. doi: 10.1016/j.apsb.2020.02.015. Epub 2020 Mar 5. Acta Pharm Sin B. 2020. PMID: 32642407 Free PMC article.
-
Matrix metalloproteinase-2 or -9 deletions protect against hemorrhagic transformation during early stage of cerebral ischemia and reperfusion.Neuroscience. 2012 Jun 14;212:180-9. doi: 10.1016/j.neuroscience.2012.03.036. Epub 2012 Apr 19. Neuroscience. 2012. PMID: 22521821 Free PMC article.
-
Hypoxia-induced neuroinflammatory white-matter injury reduced by minocycline in SHR/SP.J Cereb Blood Flow Metab. 2015 Jul;35(7):1145-53. doi: 10.1038/jcbfm.2015.21. Epub 2015 Feb 25. J Cereb Blood Flow Metab. 2015. PMID: 25712499 Free PMC article.
-
The alterations of matrix metalloproteinase-9 in mouse brainstem during herpes simplex virus type 1-induced facial palsy.J Mol Neurosci. 2013 Nov;51(3):703-9. doi: 10.1007/s12031-013-0051-3. Epub 2013 Jul 2. J Mol Neurosci. 2013. PMID: 23817985
References
-
- Ahn MY, Zhang ZG, Tsang W, Chopp M. Endogenous plasminogen activator expression after embolic focal cerebral ischemia in mice. Brain Res. 1999;837:169–176. - PubMed
-
- Asahi M, Asahi K, Wang X, Lo EH. Reduction of tissue plasminogen activator induced hemorrhage and brain injury by free radical spin trapping after focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2000a;20:452–457. - PubMed
-
- Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role of matrix metalloproteinase 9 in focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000b;20:1681–1690. - PubMed
-
- Bartus RT, Elliott PJ, Hayward NJ, Dean RL, Harbeson S, Straub JA, Li Z, Powers JC. Calpain as a novel target for treating acute neurodegenerative disorders. Neurol Res. 1995;17:249–258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
