High extracellular levels of Mycobacterium tuberculosis glutamine synthetase and superoxide dismutase in actively growing cultures are due to high expression and extracellular stability rather than to a protein-specific export mechanism
- PMID: 11553579
- PMCID: PMC98770
- DOI: 10.1128/IAI.69.10.6348-6363.2001
High extracellular levels of Mycobacterium tuberculosis glutamine synthetase and superoxide dismutase in actively growing cultures are due to high expression and extracellular stability rather than to a protein-specific export mechanism
Abstract
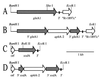
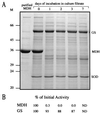
Glutamine synthetase (GS) and superoxide dismutase (SOD), large multimeric enzymes that are thought to play important roles in the pathogenicity of Mycobacterium tuberculosis, are among the bacterium's major culture filtrate proteins in actively growing cultures. Although these proteins lack a leader peptide, their presence in the extracellular medium during early stages of growth suggested that they might be actively secreted. To understand their mechanism of export, we cloned the homologous genes (glnA1 and sodA) from the rapid-growing, nonpathogenic Mycobacterium smegmatis, generated glnA1 and sodA mutants of M. smegmatis by allelic exchange, and quantitated expression and export of both mycobacterial and nonmycobacterial GSs and SODs in these mutants. We also quantitated expression and export of homologous and heterologous SODs from M. tuberculosis. When each of the genes was expressed from a multicopy plasmid, M. smegmatis exported comparable proportions of both the M. tuberculosis and M. smegmatis GSs (in the glnA1 strain) or SODs (in the sodA strain), in contrast to previous observations in wild-type strains. Surprisingly, recombinant M. smegmatis and M. tuberculosis strains even exported nonmycobacterial SODs. To determine the extent to which export of these large, leaderless proteins is expression dependent, we constructed a recombinant M. tuberculosis strain expressing green fluorescent protein (GFP) at high levels and a recombinant M. smegmatis strain coexpressing the M. smegmatis GS, M. smegmatis SOD, and M. tuberculosis BfrB (bacterioferritin) at high levels. The recombinant M. tuberculosis strain exported GFP even in early stages of growth and at proportions very similar to those of the endogenous M. tuberculosis GS and SOD. Similarly, the recombinant M. smegmatis strain exported bacterioferritin, a large (approximately 500-kDa), leaderless, multimeric protein, in proportions comparable to GS and SOD. In contrast, high-level expression of the large, leaderless, multimeric protein malate dehydrogenase did not lead to extracellular accumulation because the protein was highly unstable extracellularly. These findings indicate that, contrary to expectations, export of M. tuberculosis GS and SOD in actively growing cultures is not due to a protein-specific export mechanism, but rather to bacterial leakage or autolysis, and that the extracellular abundance of these enzymes is simply due to their high level of expression and extracellular stability. The same determinants likely explain the presence of other leaderless proteins in the extracellular medium of actively growing M. tuberculosis cultures.
Figures




Similar articles
-
Export of recombinant Mycobacterium tuberculosis superoxide dismutase is dependent upon both information in the protein and mycobacterial export machinery. A model for studying export of leaderless proteins by pathogenic mycobacteria.J Biol Chem. 1999 Feb 12;274(7):4281-92. doi: 10.1074/jbc.274.7.4281. J Biol Chem. 1999. PMID: 9933629
-
All four Mycobacterium tuberculosis glnA genes encode glutamine synthetase activities but only GlnA1 is abundantly expressed and essential for bacterial homeostasis.Mol Microbiol. 2005 Nov;58(4):1157-72. doi: 10.1111/j.1365-2958.2005.04899.x. Mol Microbiol. 2005. PMID: 16262797
-
Poly-L-glutamate/glutamine synthesis in the cell wall of Mycobacterium bovis is regulated in response to nitrogen availability.BMC Microbiol. 2013 Oct 11;13:226. doi: 10.1186/1471-2180-13-226. BMC Microbiol. 2013. PMID: 24112767 Free PMC article.
-
Mycobacterium smegmatis: The Vanguard of Mycobacterial Research.J Bacteriol. 2023 Jan 26;205(1):e0033722. doi: 10.1128/jb.00337-22. Epub 2023 Jan 4. J Bacteriol. 2023. PMID: 36598232 Free PMC article. Review.
-
Production of recombinant proteins in Mycobacterium smegmatis for structural and functional studies.Protein Sci. 2015 Jan;24(1):1-10. doi: 10.1002/pro.2584. Epub 2014 Nov 13. Protein Sci. 2015. PMID: 25303009 Free PMC article. Review.
Cited by
-
The role of GlnD in ammonia assimilation in Mycobacterium tuberculosis.Tuberculosis (Edinb). 2007 Jul;87(4):384-90. doi: 10.1016/j.tube.2006.12.003. Epub 2007 Feb 15. Tuberculosis (Edinb). 2007. PMID: 17303474 Free PMC article.
-
Regulation of the Mycobacterium tuberculosis mce1 operon.J Bacteriol. 2006 Jan;188(2):441-9. doi: 10.1128/JB.188.2.441-449.2006. J Bacteriol. 2006. PMID: 16385033 Free PMC article.
-
Host-adaptation of Francisella tularensis alters the bacterium's surface-carbohydrates to hinder effectors of innate and adaptive immunity.PLoS One. 2011;6(7):e22335. doi: 10.1371/journal.pone.0022335. Epub 2011 Jul 22. PLoS One. 2011. PMID: 21799828 Free PMC article.
-
Carbon flux rerouting during Mycobacterium tuberculosis growth arrest.Mol Microbiol. 2010 Dec;78(5):1199-215. doi: 10.1111/j.1365-2958.2010.07399.x. Epub 2010 Oct 6. Mol Microbiol. 2010. PMID: 21091505 Free PMC article.
-
Identifying vulnerable pathways in Mycobacterium tuberculosis by using a knockdown approach.Appl Environ Microbiol. 2011 Jul;77(14):5040-3. doi: 10.1128/AEM.02880-10. Epub 2011 Jun 3. Appl Environ Microbiol. 2011. PMID: 21642404 Free PMC article.
References
-
- Alcendor D J, Chapman G D, Beaman B L. Isolation, sequencing and expression of the superoxide dismutase-encoding gene (sod) of Nocardia asteroides strain GUH-2. Gene. 1995;164:143–147. - PubMed
-
- Alito A, Romano M I, Bigi F, Zumarraga M, Cataldi A. Antigenic characterization of mycobacteria from South American wild seals. Vet Microbiol. 1999;68:293–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

