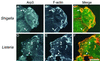
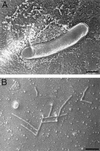
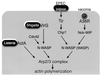
Molecular basis of the intracellular spreading of Shigella
- PMID: 11553531
- PMCID: PMC98722
- DOI: 10.1128/IAI.69.10.5959-5966.2001
Molecular basis of the intracellular spreading of Shigella
Figures
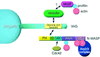
Similar articles
-
[Studies on the actin-based spreading of Shigella].Nihon Saikingaku Zasshi. 2002 May;57(2):443-52. Nihon Saikingaku Zasshi. 2002. PMID: 12048885 Review. Japanese. No abstract available.
-
Quantification of Shigella IcsA required for bacterial actin polymerization.Cell Motil Cytoskeleton. 2002 Apr;51(4):187-96. doi: 10.1002/cm.10024. Cell Motil Cytoskeleton. 2002. PMID: 11977093
-
Diversion of cytoskeletal processes by Shigella during invasion of epithelial cells.Microbes Infect. 2000 Jun;2(7):813-9. doi: 10.1016/s1286-4579(00)90366-6. Microbes Infect. 2000. PMID: 10955962 Review.
-
Analysis of the mechanisms of Salmonella-induced actin assembly during invasion of host cells and intracellular replication.Cell Microbiol. 2004 Nov;6(11):1041-55. doi: 10.1111/j.1462-5822.2004.00417.x. Cell Microbiol. 2004. PMID: 15469433
-
Capping of actin filaments by vinculin activated by the Shigella IpaA carboxyl-terminal domain.FEBS Lett. 2007 Mar 6;581(5):853-7. doi: 10.1016/j.febslet.2007.01.057. Epub 2007 Feb 2. FEBS Lett. 2007. PMID: 17289036
Cited by
-
Mutagenesis of the Shigella flexneri autotransporter IcsA reveals novel functional regions involved in IcsA biogenesis and recruitment of host neural Wiscott-Aldrich syndrome protein.J Bacteriol. 2008 Jul;190(13):4666-76. doi: 10.1128/JB.00093-08. Epub 2008 May 2. J Bacteriol. 2008. PMID: 18456802 Free PMC article.
-
The immunogenic SigA enterotoxin of Shigella flexneri 2a binds to HEp-2 cells and induces fodrin redistribution in intoxicated epithelial cells.PLoS One. 2009 Dec 9;4(12):e8223. doi: 10.1371/journal.pone.0008223. PLoS One. 2009. PMID: 20011051 Free PMC article. Clinical Trial.
-
Initial steps of Shigella infection depend on the cholesterol/sphingolipid raft-mediated CD44-IpaB interaction.EMBO J. 2002 Sep 2;21(17):4449-57. doi: 10.1093/emboj/cdf457. EMBO J. 2002. PMID: 12198147 Free PMC article.
-
Cortactin and Crk cooperate to trigger actin polymerization during Shigella invasion of epithelial cells.J Cell Biol. 2004 Jul 19;166(2):225-35. doi: 10.1083/jcb.200402073. J Cell Biol. 2004. PMID: 15263018 Free PMC article.
-
Identification of Shigella flexneri IcsA residues affecting interaction with N-WASP, and evidence for IcsA-IcsA co-operative interaction.PLoS One. 2013;8(2):e55152. doi: 10.1371/journal.pone.0055152. Epub 2013 Feb 6. PLoS One. 2013. PMID: 23405119 Free PMC article.
References
-
- Bear J E, Loureiro J J, Libova I, Fassler R, Wehland J, Gertler F B. Negative regulation of fibroblast motility by Ena/VASP proteins. Cell. 2000;101:717–728. - PubMed
-
- Cameron L A, Giardini P A, Soo F S, Theriot J A. Secrets of actin-based motility revealed by a bacterial pathogen. Nat Rev Mol Cell Biol. 2000;1:110–119. - PubMed
-
- Chakraborty T, Ebel F, Domann E, Niebuhr K, Gerstel B, Pistor S, Temm-Grove C J, Jockusch B M, Reinhard M, Walter U, Wehland J. A focal adhesion factor directly linking intracellularly motile Listeria monocytogenes and Listeria ivanovii to the actin-based cytoskeleton of mammalian cells. EMBO J. 1995;14:1314–1321. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

