Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease
- PMID: 11518734
- PMCID: PMC209401
- DOI: 10.1172/JCI12821
Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease
Abstract
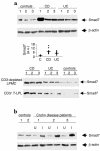
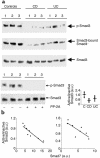
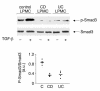
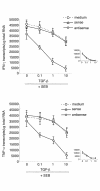
TGF-beta1 functions as a negative regulator of T cell immune responses, signaling to target cells using the Smad family of proteins. We show here that Smad7, an inhibitor of TGF-beta1 signaling, is overexpressed in inflammatory bowel disease (IBD) mucosa and purified mucosal T cells. Both whole tissue and isolated cells exhibit defective signaling through this pathway, as measured by phospho-Smad3 immunoreactivity. Specific antisense oligonucleotides for Smad7 reduce Smad7 protein expression in cells isolated from patients with IBD, permitting the cells to respond to exogenous TGF-beta1. TGF-beta1 cannot inhibit proinflammatory cytokine production in isolated lamina propria mononuclear cells from patients with Crohn disease (CD), but inhibition of Smad7 restores TGF-beta1 signaling and enables TGF-beta1 to inhibit cytokine production. In inflamed mucosal tissue explants from patients with CD, inhibition of Smad7 also restores p-Smad3 and decreases proinflammatory cytokine production, an effect that is partially blocked by anti-TGF-beta1. These results show that Smad7 blockade of TGF-beta1 signaling helps maintain the chronic production of proinflammatory cytokines that drives the inflammatory process in IBD and that inhibition of Smad7 enables endogenous TGF-beta to downregulate this response.
Figures


Comment in
-
TGF-beta/Smad signaling defects in inflammatory bowel disease: mechanisms and possible novel therapies for chronic inflammation.J Clin Invest. 2001 Aug;108(4):523-6. doi: 10.1172/JCI13863. J Clin Invest. 2001. PMID: 11518725 Free PMC article. No abstract available.
Similar articles
-
Inhibition of TGF-beta signaling by IL-15: a new role for IL-15 in the loss of immune homeostasis in celiac disease.Gastroenterology. 2007 Mar;132(3):994-1008. doi: 10.1053/j.gastro.2006.12.025. Epub 2006 Dec 16. Gastroenterology. 2007. PMID: 17324400
-
Inhibition of Smad7 with a specific antisense oligonucleotide facilitates TGF-beta1-mediated suppression of colitis.Gastroenterology. 2006 Dec;131(6):1786-98. doi: 10.1053/j.gastro.2006.09.016. Epub 2006 Sep 19. Gastroenterology. 2006. PMID: 17087939
-
Transforming growth factor-beta-induced inhibition of myogenesis is mediated through Smad pathway and is modulated by microtubule dynamic stability.Circ Res. 2004 Mar 19;94(5):617-25. doi: 10.1161/01.RES.0000118599.25944.D5. Epub 2004 Jan 22. Circ Res. 2004. PMID: 14739161
-
Smad7 in TGF-beta-mediated negative regulation of gut inflammation.Trends Immunol. 2004 Oct;25(10):513-7. doi: 10.1016/j.it.2004.07.008. Trends Immunol. 2004. PMID: 15364052 Review.
-
TGF-Beta signaling manipulation as potential therapy for IBD.Curr Drug Targets. 2013 Nov;14(12):1400-4. doi: 10.2174/13894501113149990157. Curr Drug Targets. 2013. PMID: 23489130 Review.
Cited by
-
T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells.J Exp Med. 2005 Mar 7;201(5):737-46. doi: 10.1084/jem.20040685. J Exp Med. 2005. PMID: 15753207 Free PMC article.
-
Smad7 Controls Immunoregulatory PDL2/1-PD1 Signaling in Intestinal Inflammation and Autoimmunity.Cell Rep. 2019 Sep 24;28(13):3353-3366.e5. doi: 10.1016/j.celrep.2019.07.065. Cell Rep. 2019. PMID: 31553906 Free PMC article.
-
Small intestinal CD8+TCRgammadelta+NKG2A+ intraepithelial lymphocytes have attributes of regulatory cells in patients with celiac disease.J Clin Invest. 2008 Jan;118(1):281-93. doi: 10.1172/JCI30989. J Clin Invest. 2008. PMID: 18064301 Free PMC article.
-
Mechanisms of intestinal inflammation and development of associated cancers: lessons learned from mouse models.Mutat Res. 2010 Jul-Sep;705(1):40-59. doi: 10.1016/j.mrrev.2010.03.001. Epub 2010 Mar 16. Mutat Res. 2010. PMID: 20298806 Free PMC article. Review.
-
Diversity of Intestinal Macrophages in Inflammatory Bowel Diseases.Front Immunol. 2015 Dec 7;6:613. doi: 10.3389/fimmu.2015.00613. eCollection 2015. Front Immunol. 2015. PMID: 26697009 Free PMC article. Review.
References
-
- Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. - PubMed
-
- Letterio JJ, Roberts AB. Regulation of immune responses by TGF-β. Annu Rev Immunol. 1998;16:137–161. - PubMed
-
- Piek E, Heldin C-H, ten Dijke P. Specificity, diversity, and regulation in TGF-β superfamily signaling. FASEB J. 1999;13:2105–2124. - PubMed
-
- Heldin C-H, Kohei M, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

