Cdc42-dependent modulation of tight junctions and membrane protein traffic in polarized Madin-Darby canine kidney cells
- PMID: 11514615
- PMCID: PMC58593
- DOI: 10.1091/mbc.12.8.2257
Cdc42-dependent modulation of tight junctions and membrane protein traffic in polarized Madin-Darby canine kidney cells
Abstract
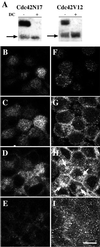
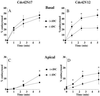
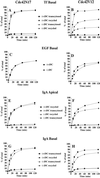
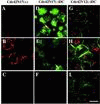
Polarized epithelial cells maintain the asymmetric composition of their apical and basolateral membrane domains by at least two different processes. These include the regulated trafficking of macromolecules from the biosynthetic and endocytic pathway to the appropriate membrane domain and the ability of the tight junction to prevent free mixing of membrane domain-specific proteins and lipids. Cdc42, a Rho family GTPase, is known to govern cellular polarity and membrane traffic in several cell types. We examined whether this protein regulated tight junction function in Madin-Darby canine kidney cells and pathways that direct proteins to the apical and basolateral surface of these cells. We used Madin-Darby canine kidney cells that expressed dominant-active or dominant-negative mutants of Cdc42 under the control of a tetracycline-repressible system. Here we report that expression of dominant-active Cdc42V12 or dominant-negative Cdc42N17 altered tight junction function. Expression of Cdc42V12 slowed endocytic and biosynthetic traffic, and expression of Cdc42N17 slowed apical endocytosis and basolateral to apical transcytosis but stimulated biosynthetic traffic. These results indicate that Cdc42 may modulate multiple cellular pathways required for the maintenance of epithelial cell polarity.
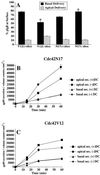
Figures




Similar articles
-
Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells.Nat Cell Biol. 1999 May;1(1):8-13. doi: 10.1038/8977. Nat Cell Biol. 1999. PMID: 10559857
-
RhoA, Rac1, and Cdc42 exert distinct effects on epithelial barrier via selective structural and biochemical modulation of junctional proteins and F-actin.Am J Physiol Cell Physiol. 2004 Aug;287(2):C327-35. doi: 10.1152/ajpcell.00087.2004. Epub 2004 Mar 24. Am J Physiol Cell Physiol. 2004. PMID: 15044152
-
Modulation of endocytic traffic in polarized Madin-Darby canine kidney cells by the small GTPase RhoA.Mol Biol Cell. 1999 Dec;10(12):4369-84. doi: 10.1091/mbc.10.12.4369. Mol Biol Cell. 1999. PMID: 10588664 Free PMC article.
-
Epithelial junctions and Rho family GTPases: the zonular signalosome.Small GTPases. 2014;5(4):1-15. doi: 10.4161/21541248.2014.973760. Small GTPases. 2014. PMID: 25483301 Free PMC article. Review.
-
The interdependence of the Rho GTPases and apicobasal cell polarity.Small GTPases. 2014;5(2):10. doi: 10.4161/21541248.2014.973768. Small GTPases. 2014. PMID: 25469537 Free PMC article. Review.
Cited by
-
Rab10 regulates membrane transport through early endosomes of polarized Madin-Darby canine kidney cells.Mol Biol Cell. 2006 Jul;17(7):3156-75. doi: 10.1091/mbc.e05-08-0799. Epub 2006 Apr 26. Mol Biol Cell. 2006. PMID: 16641372 Free PMC article.
-
The Cdc42 GEF Intersectin 2 controls mitotic spindle orientation to form the lumen during epithelial morphogenesis.J Cell Biol. 2010 May 17;189(4):725-38. doi: 10.1083/jcb.201002047. J Cell Biol. 2010. PMID: 20479469 Free PMC article.
-
Regulation of protein transport from the Golgi complex to the endoplasmic reticulum by CDC42 and N-WASP.Mol Biol Cell. 2002 Mar;13(3):866-79. doi: 10.1091/mbc.01-12-0579. Mol Biol Cell. 2002. PMID: 11907268 Free PMC article.
-
Simian virus 40 small tumor antigen induces deregulation of the actin cytoskeleton and tight junctions in kidney epithelial cells.J Virol. 2003 Mar;77(5):2807-18. doi: 10.1128/jvi.77.5.2807-2818.2003. J Virol. 2003. PMID: 12584304 Free PMC article.
-
Mechanical checkpoint for persistent cell polarization in adhesion-naive fibroblasts.Biophys J. 2014 Jul 15;107(2):324-335. doi: 10.1016/j.bpj.2014.05.041. Biophys J. 2014. PMID: 25028874 Free PMC article.
References
-
- Apodaca G. Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic. 2001;2:149–159. - PubMed
-
- Apodaca G, Aroeti B, Tang K, Mostov KE. Brefeldin-A inhibits the delivery of the polymeric immunoglobulin receptor to the basolateral surface of MDCK cells. J Biol Chem. 1993;268:20380–20385. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

