Subunit heterogeneity of cytoplasmic dynein: Differential expression of 14 kDa dynein light chains in rat hippocampus
- PMID: 11466421
- PMCID: PMC3880687
- DOI: 10.1523/JNEUROSCI.21-15-05501.2001
Subunit heterogeneity of cytoplasmic dynein: Differential expression of 14 kDa dynein light chains in rat hippocampus
Abstract
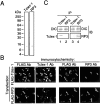
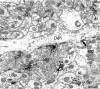
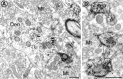
Cytoplasmic dynein is a multi-subunit protein complex in which each subunit is encoded by a few genes. How these subunit isoforms are assembled and regulated to mediate the diverse functions of cytoplasmic dynein is unknown. We previously have shown that two highly conserved 14 kDa dynein light chains, Tctex-1 and RP3, have different cargo-binding abilities. In this report, coimmunoprecipitation revealed that Tctex-1 and RP3 were present in mutually exclusive dynein complexes of brain. Two specific antibodies were used to examine the localization of these two dynein light chains in adult rat hippocampal formation and cerebral cortex. By light microscopy, Tctex-1 and RP3 immunoreactivities exhibited distinct and almost complementary distribution patterns in both brain regions. In hippocampal formation, Tctex-1 immunoreactivity was most enriched in somata of newly generated granule cells and scant in the mature granule and pyramidal cell somata. In contrast, RP3 immunoreactivity was abundant in pyramidal and granule cell somata. Ultrastructural analysis of the dentate gyrus revealed both dynein light chains were associated with various membranous organelles that often were affiliated with microtubules. In addition, Tctex-1 and RP3 immunoreactivities were preferentially and highly enriched on membranous organelles and/or vesicles of axon terminals and dendritic spines, respectively. These results suggest that dynein complexes with different subunit composition, and possibly function, are expressed differentially in a spatially and temporally regulated manner. Furthermore, Tctex-1 and RP3 may play important roles in synaptic functions.
Figures






Similar articles
-
Cytoplasmic dynein contains a family of differentially expressed light chains.Biochemistry. 1998 Oct 27;37(43):15033-41. doi: 10.1021/bi9810813. Biochemistry. 1998. PMID: 9790665
-
Cytoplasmic dynein regulation by subunit heterogeneity and its role in apical transport.J Cell Biol. 2001 Jun 25;153(7):1499-509. doi: 10.1083/jcb.153.7.1499. J Cell Biol. 2001. PMID: 11425878 Free PMC article.
-
Interactions of cytoplasmic dynein light chains Tctex-1 and LC8 with the intermediate chain IC74.Biochemistry. 2002 Apr 2;41(13):4302-11. doi: 10.1021/bi011970h. Biochemistry. 2002. PMID: 11914076
-
Distinct cytoplasmic dynein complexes are transported by different mechanisms in axons.Biochim Biophys Acta. 2000 Mar 17;1496(1):76-88. doi: 10.1016/s0167-4889(00)00010-0. Biochim Biophys Acta. 2000. PMID: 10722878 Review.
-
Cytoplasmic dynein subunit heterogeneity: implications for axonal transport.J Neurocytol. 2000 Nov-Dec;29(11-12):819-29. doi: 10.1023/a:1010995408343. J Neurocytol. 2000. PMID: 11466473 Review.
Cited by
-
The role of the cytoskeleton in the life cycle of viruses and intracellular bacteria: tracks, motors, and polymerization machines.Curr Drug Targets Infect Disord. 2002 Sep;2(3):247-64. doi: 10.2174/1568005023342407. Curr Drug Targets Infect Disord. 2002. PMID: 12462128 Free PMC article.
-
Binding of microtubule-associated protein 1B to LIS1 affects the interaction between dynein and LIS1.Biochem J. 2005 Jul 15;389(Pt 2):333-41. doi: 10.1042/BJ20050244. Biochem J. 2005. PMID: 15762842 Free PMC article.
-
Phosphorylation of DYNLT1 at serine 82 regulates microtubule stability and mitochondrial permeabilization in hypoxia.Mol Cells. 2013 Oct;36(4):322-32. doi: 10.1007/s10059-013-0114-x. Epub 2013 Oct 22. Mol Cells. 2013. PMID: 24170091 Free PMC article.
-
Transgenic mouse models for studying adult neurogenesis.Front Biol (Beijing). 2016 Jun;11(3):151-167. doi: 10.1007/s11515-016-1405-3. Epub 2016 Jun 28. Front Biol (Beijing). 2016. PMID: 28473846 Free PMC article.
-
The dynein light chain Tctex-1 has a dynein-independent role in actin remodeling during neurite outgrowth.Dev Cell. 2005 Jul;9(1):75-86. doi: 10.1016/j.devcel.2005.04.003. Dev Cell. 2005. PMID: 15992542 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
